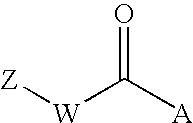
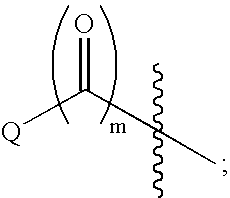
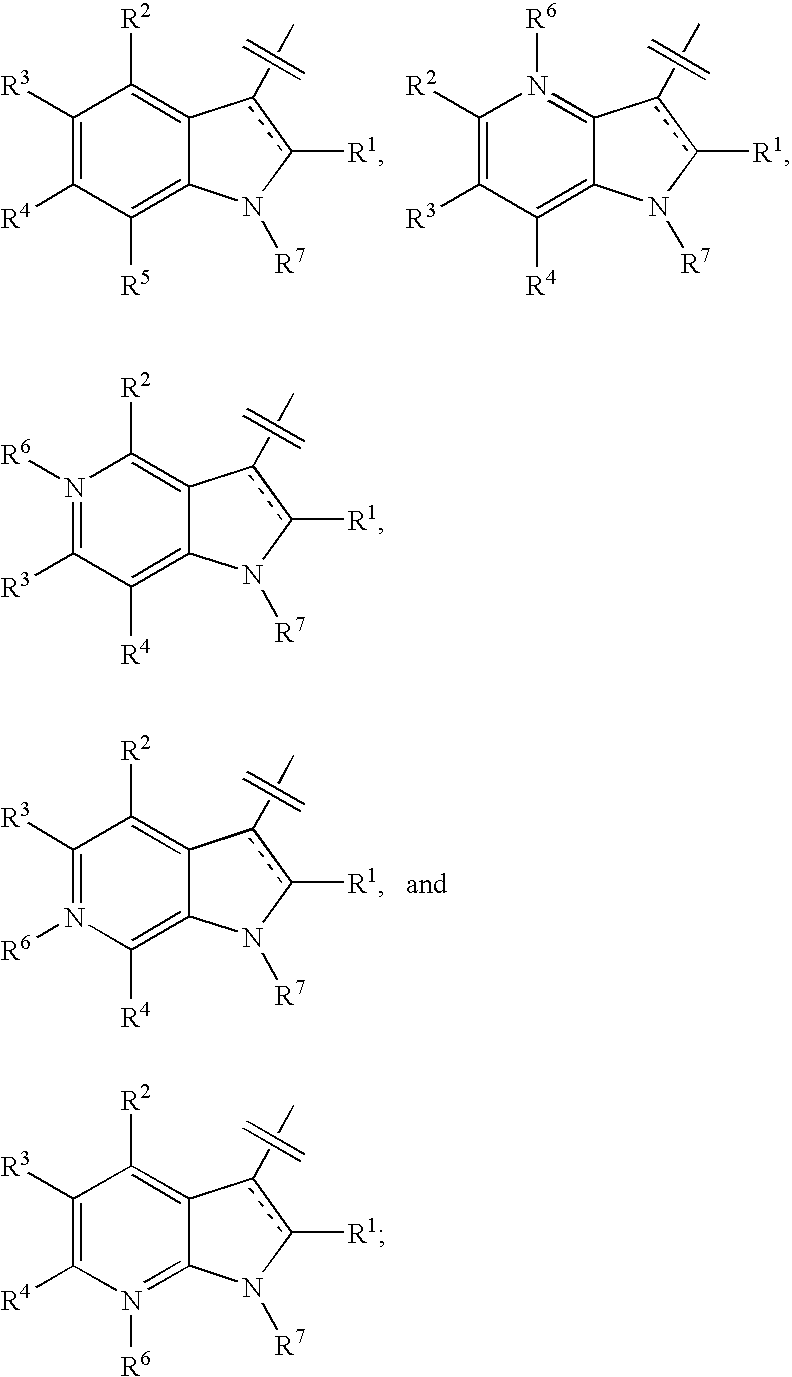
Indole, azaindole and related heterocyclic pyrrolidine derivatives
a heterocyclic pyrrolidine and derivative technology, applied in the field of compounds with drug and bioaffecting properties, can solve the problems of 30 to 50% of patients ultimately failing combination drug therapies, each of these drugs can only transiently restrain viral replication if used alone, and the effect of viremia and disease progression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-(1-Benzoyl-pyrrolidin-3-ylmethyl)-2-(4-fluoro-1H-indol-3-yl)-2-oxo-acetamide
[0269]
[0270] The acid chloride (1 mmol, 225 mg) was added to a solution of the amine hydrochloride (1 mmol, 240 mg) and diisopropyl ethyl amine (10 mmol, 1.74 mL) in 5 mL of anhydrous THF under a nitrogen atmosphere. The reaction was stirred for 16 h at ambient temperature and then poured into ethyl acetate. The organic layer was washed with water and then sat aq NaCl and then dried over anhydrous magnesium sulfate. Filtration and concentration in vacuo provided a crude product which was purified by flash chromatography over silica gel to provide 208 mg of the desired N-(1-Benzoyl-pyrrolidin-3-ylmethyl)-2-(4-fluoro-1H-indol-3-yl)-2-oxo-acetamide.
[0271] 1H NMR (500 MHz, CD3OD): 8.69 (s), 8.56 (s), 1H. 7.55˜7.40 (m, 5H), 7.31˜7.20 (m, 2H), 6.96˜6.87 (m, 1H), 3.81˜3.29 (m, 6H), 2.70˜1.73 (m, 3H).
[0272] LC / MS: (ES+) m / z (M+H)+=394, RT=1.06.
example 2
{1-[2-(4-Fluoro-1H-indol-3-yl)-2-oxo-acetyl]-pyrrolidin-3-ylmethyl}-carbamic acid tert-butyl ester
[0273]
[0274] The acid chloride (2 mmol, 453 mg) was added to a solution of the amine hydrochloride (2 mmol, 400 mg) and diisopropyl ethyl amine (4 mmol, 0.7 mL) in 12 mL of anhydrous THF under a nitrogen atmosphere. The reaction was stirred for 18 h at ambient temperature and then poured into ethyl acetate. The organic layer was washed with water and then sat aq NaCl and then dried over anhydrous magnesium sulfate. Filtration and concentration in vacuo provided a crude product which was purified by flash chromatography over silica gel to provide 475 mg of the desired 3-{[2-(4-Fluoro-1H-indol-3-yl)-2-oxo-acetylamino]-methyl}-pyrrolidine-1-carboxylic acid tert-butyl ester.
[0275] 1H NMR (500 MHz, CD3OD): 8.17(s), 8.14(S), 1H. 7.32˜7.24(m, 2H), 6.97˜6.93(m, 1H), 3.73˜3.04(m, 6H), 2.46˜2.43(m, 1H), 2.09˜2.01(m, 1H), 1.74˜1.73(m, 1H), 1.44(s, 9H).
Intermediate 8
[0276]
[0277] The carbamate wa...
example 3
N-{1-[2-(4-Fluoro-1H-indol-3-yl)-2-oxo-acetyl]-pyrrolidin-3-ylmethyl}-benzamide and Example 4N-{1-[2-(1-Benzoyl-4-fluoro-1H-indol-3-yl)-2-oxo-acetyl]-pyrrolidin-3-ylmethyl}-benzamide
[0278]
[0279] Benzoyl chloride (0.46 mmol, 54 μL) and then diisopropyl ethyl amine (0.92 mmol, 0.16 mL) were added to a stirring solution of amine hydrochloride (0.46 mmol, 150 mg) in 5 mL of THF under an atmosphere of nitrogen at ambient temperature. The reaction was stirred for 18 h and then the THF was removed in vacuo. The residue was dissolved in ethyl acetate and washed with water and then sat aq NaCl. The organic extract was dried, filtered, concentrated, and purified via flash chromatography to the desired product of example 3:
[0280] 1H NMR (500 MHz, CD3OD): 8.18 (s), 8.14 (s), 1H. 7.85˜7.82 (m, 1H), 7.72˜7.68 (m, 1H), 7.54˜7.24 (m, 5H), 6.97˜6.90 (m, 1H), 3.80˜3.29 (m, 6H), 2.67˜2.61 (m, 1H), 2.20˜2.05(m, 1H), 1.87˜1.81(m, 1H).
[0281] LC / MS: (ES+) m / z (M+H)+=394, RT=1.74.
and the bis benzylate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| Temperatures | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com