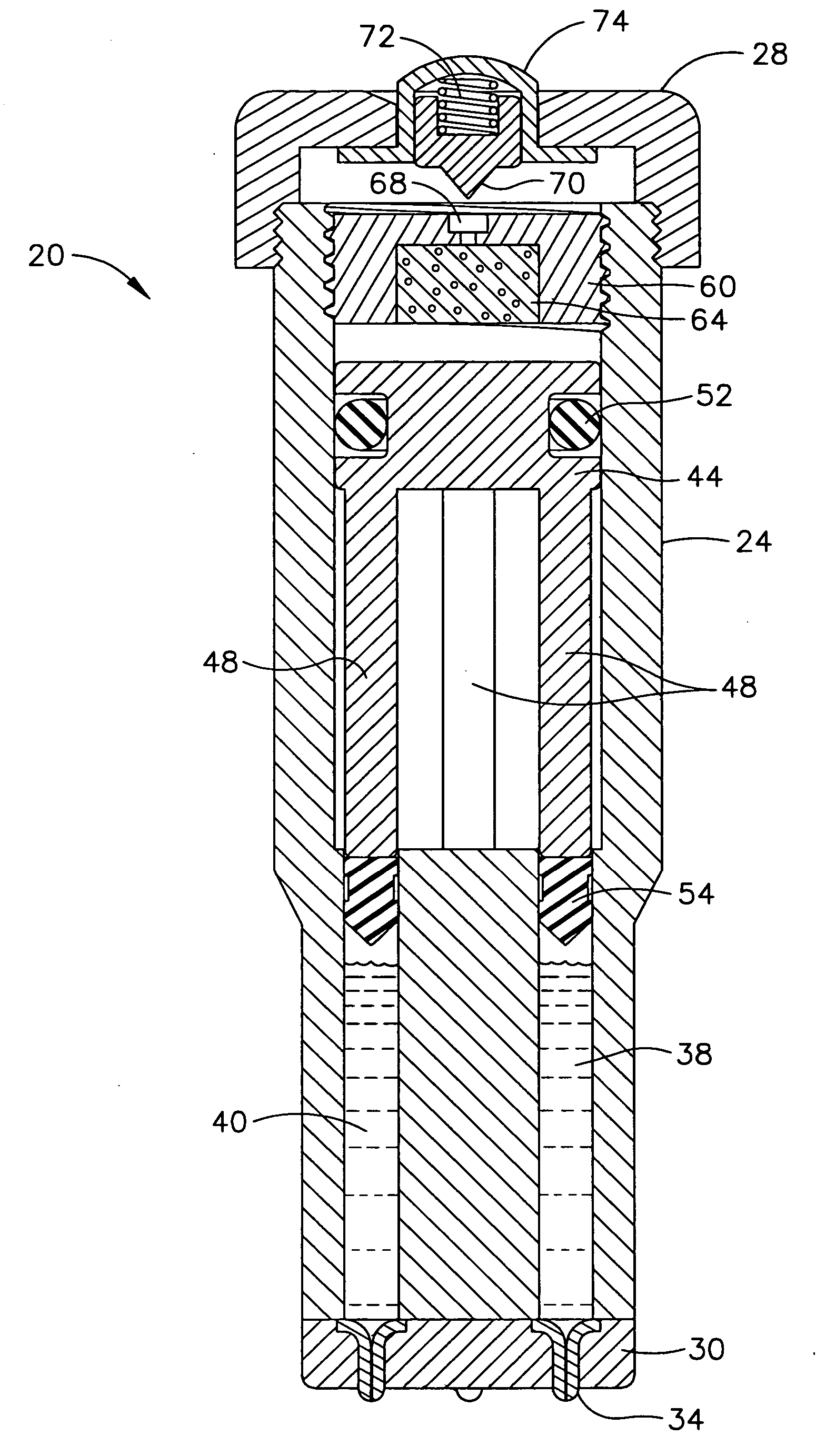
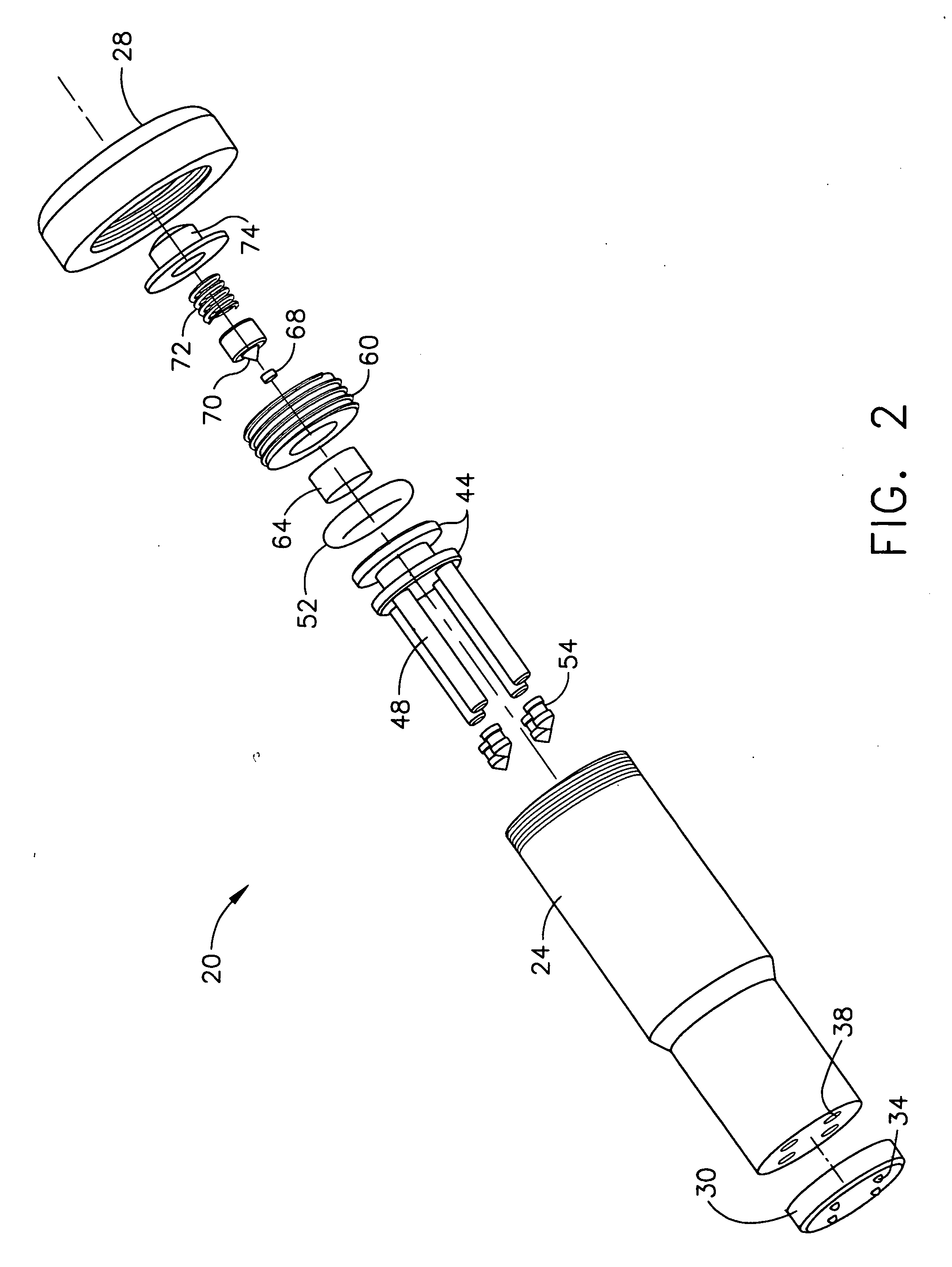
Despite the continual advances in medical technology, particularly in the treatment of various diseases such as
heart disease,
vascular disease,
ophthalmic disease,
cancer, pain, allergies, orthopedic repair and many other diseases and conditions, there are a significant number of patients for whom conventional surgical and interventional therapies are not feasible or are insufficient to treat the
disease or condition.
Although there have been advances in needle-based
drug delivery / injection systems, these systems have significant shortcomings and disadvantages.
One such
disadvantage is that the use of a needle or other penetrating means to inject the targeted tissue area unavoidably involves making a hole into the
target site thereby causing trauma and tissue injury at the local tissue site.
Another
disadvantage with this needle penetrating and injection approach is that it is very common for a substantial amount of the injectate to leak back out or exude from the hole created by the needle or penetrating member.
Often, this leaked injectate is released systemically throughout the body or is wasted depriving the patient of the prescribed therapy or dosing amounts of the drug.
This also results in increased
treatment costs and requires more injections, time and agent in order to achieve the desired affect.
Furthermore, it is known that needle injections or penetration into the tissue can traumatize or destroy tissue cells and, as a result, increase a patient's risk of post-operative trauma, pain and discomfort at the local site and surrounding area.
This is particularly due to the difficulty in precisely controlling the penetration of the needle during injection.
The more injections or penetrations, the greater the
cell destruction and
tissue trauma that is likely experienced.
Still another
disadvantage of needle-based injections, especially where multiple injections are required, is the inability to carefully track the location of each
injection site so as to prevent the accidental delivery of drug to non-diseased tissue or repeat delivery of the drug to the same injection hole.
These types of devices could present a greater risk of releasing the agent systemically.
Additionally, with these types of devices, it is more difficult to assess the actual dosing of the target area that takes place.
Thus, these types of devices have the disadvantages of being less effective, possibly not as safe, and definitely more costly than the commonly known needle injection approaches and technology.
In order to effectively lyse the
thrombus, the thrombolytics are typically infused for many hours, even as much as a day or more, increasing the necessary length of
hospital stay and the overall cost of the procedure.
In great part, this is due to the size of the injection
stream and, thus, the size of the nozzle orifice.
There are several significant limitations with current
jet injection technology.
First, injection times associated with these conventional needle-free jet injectors are typically several seconds in length, which puts the patient at risk of laceration if they should move (e.g., flinch) or if the
injector should be jarred from the
injection site during an injection.
Second, the perceived pain is equivalent to a conventional needle and
syringe.
Third, jet injectors are prone to deliver so-called “wet injections” where
medicine leaks back out through the site of injection, a result that has given rise to concerns about accuracy of the delivered
dose.
This size resulted more from the practical limitations of
plastic injection molding for high volume commercial manufacturing than from any effort at optimizing the size for user comfort and minimization or
elimination of any “leaking” of the injected medicament.
This trade-off of sub-optimal performance for manufacturability has resulted in a marginalized product that has not enjoyed the
market acceptance it otherwise might have.
Not only is this particular arrangement complex, but it requires high delivery pressures for the contents in the ampule in a range from about 1800 to 5000 psi, with some applications in a range from about 1800 to 2300 psi.
Even though such a device does not use a needle, the negative outcome involved with using such a device and arrangement is that it is likely to cause excessive trauma to the tissue at the delivery site as well as cause unwanted and unnecessary pain and / or discomfort to the
end user or patient due to the required high delivery pressures as well as the relatively
large size of the dispersion orifices.
Accordingly, the Glines et al. device and method are not suitable for microjet delivery of drugs especially in sensitive areas of the body such as the eye, nasal passageways and mouth or other sensitive areas of the body especially those areas that are easily prone to trauma, pain and discomfort.
Accordingly, there are a number of sensitive areas in the body and
disease states that are extremely difficult to treat using local
drug delivery.
For example, there are a myriad of ophthalmic diseases that are difficult to treat and delivery of the drug to the site of disease, i.e. the eye, is often painful or psychologically uncomfortable for the patient.
For these types of disease,
systemic administration of drug commonly yields subtherapeutic drug concentrations in the eye and may have significant adverse effects.
Consequently, current treatment for diseases of the eye often involves direct injection of the medicament into the eye via a conventional needle and
syringe a painful and undesirable means of delivery for the patient.
Further, chronic treatment requires repeated injections that can result in plaque formations and scarring in the eye,
retinal detachment, and endophthalmitis.
And, it is well established that each of these approaches has its own limitations.
Additionally, ocular implants require a surgical procedure for implantation and explantation—procedures that are costly, painful, and can result in scarring to the eye.
Implants have the further limitation of physical size and the amount of drug that can be loaded or put
on board the
implant.
It is also known that
photodynamic therapy is an unproven technology whose long-term effects are not understood and may well be harmful to the
retina.
Eye drops, however, are very quickly washed out of the eye and afford only minimal delivery of the contained drug.
But, the
rapidity of the cellular turnover at the surface of the eye is believed to be limiting in the effectiveness of this means of delivery.
And, passive
diffusion cannot deliver drugs with a molecular weight greater than about 500 Daltons.
Consequently, there are currently no truly acceptable means of delivering active therapeutic agents to the eye and other sensitive areas of the body, especially the emerging macromolecules that are showing promise in the treatment of a variety of ophthalmic diseases and diseases associated with these other sensitive areas of the body.
To date, there have been no known devices or methods that provide for true needle-free delivery of drugs regardless of size of the drug molecules involved as well as provide for true needle-free delivery of drugs with minimal trauma to tissue and that are suitable for delivering drugs in sensitive areas of the body such as the eye, nasal passageways or mouth.
To date, there have also been no known devices that provide for the true needle-free delivery of drugs wherein the devices are microjet delivery devices that are simple and efficient in design and construction, low cost and easy to manufacture.
 Login to View More
Login to View More  Login to View More
Login to View More