Balloon catheter
a balloon catheter and balloon technology, applied in the field of balloon catheters, can solve the problems of limiting the extent to which the balloon can expand radially, and 40% of the expansion constrictions spontaneously collapse, etc., and achieve the effect of reducing the thickness of the wall
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
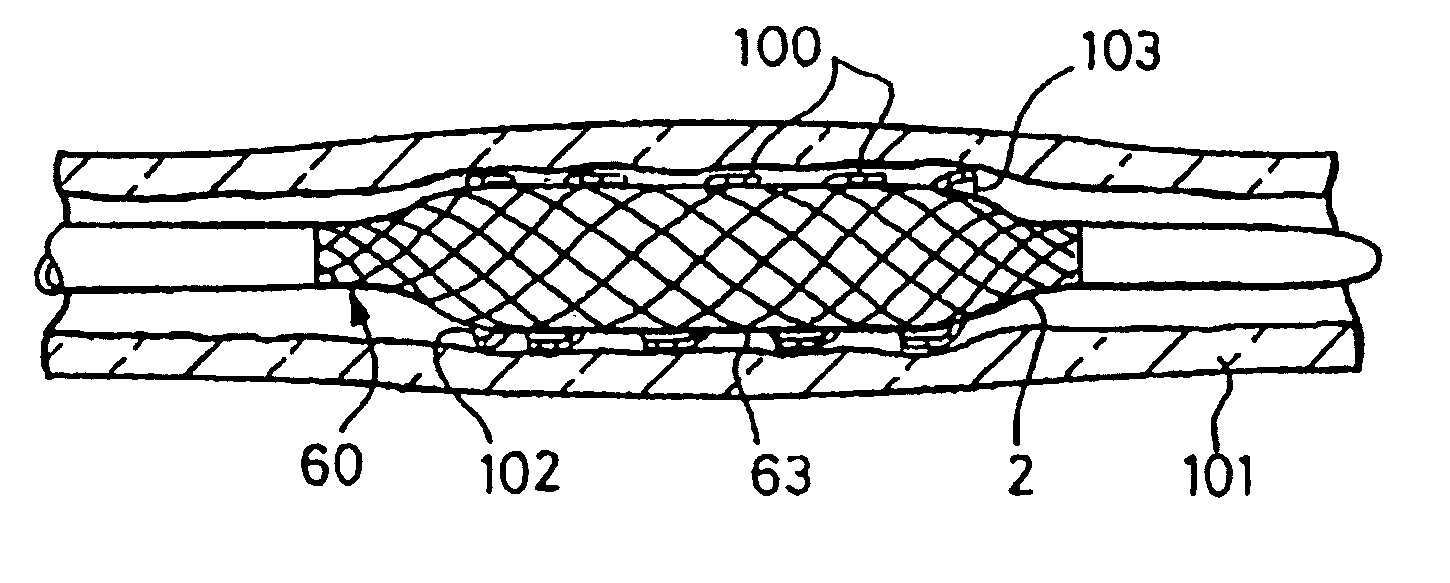
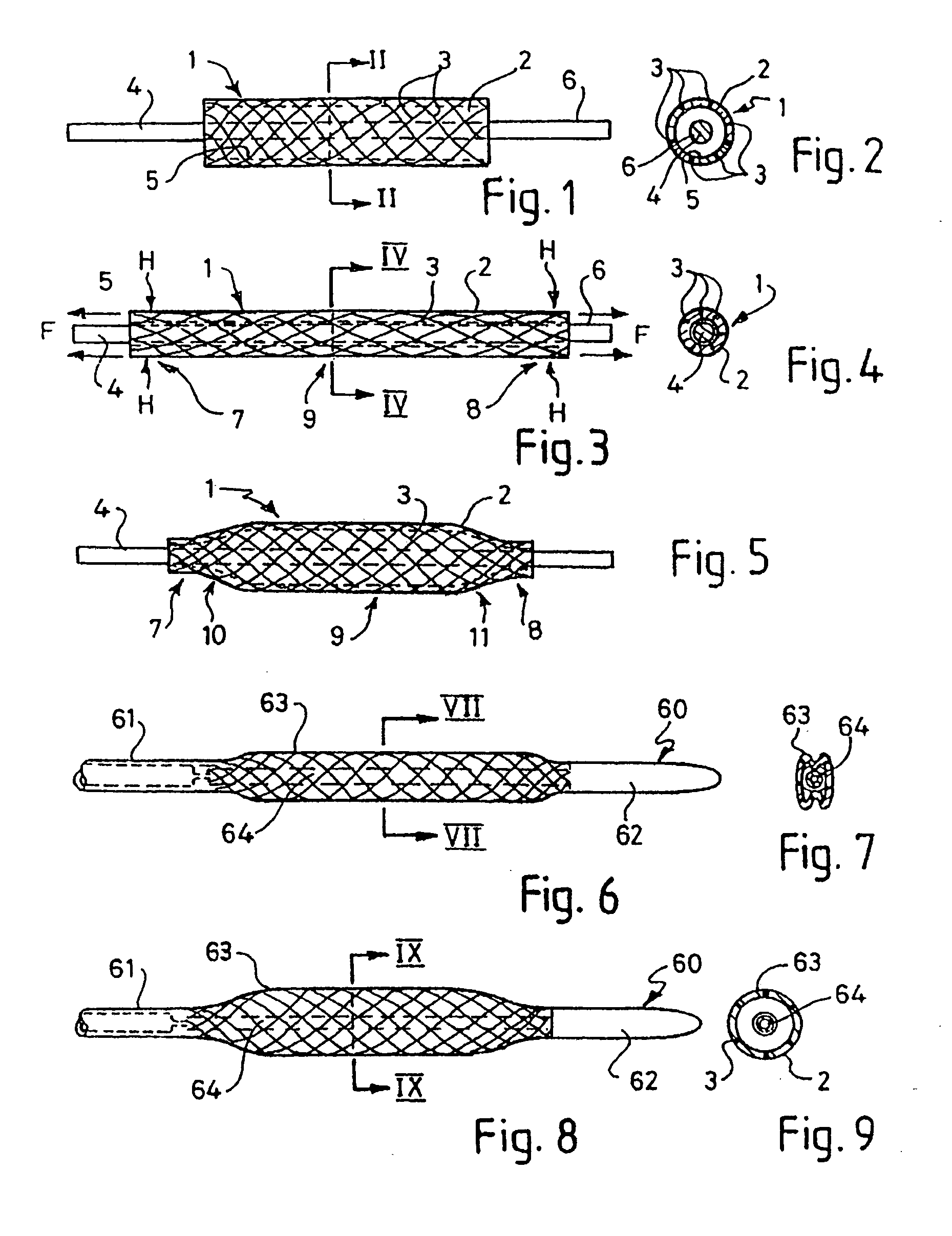
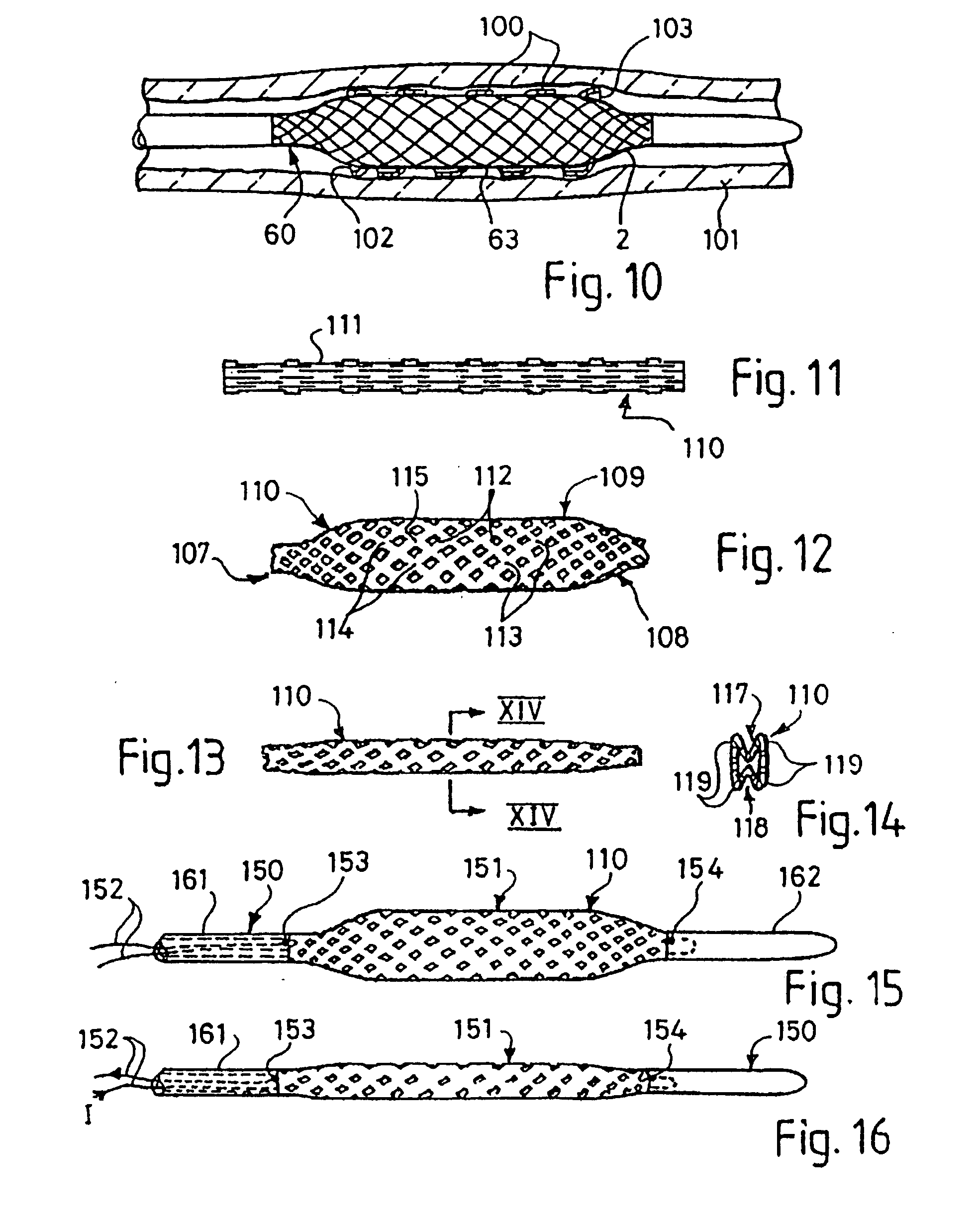
[0047] FIGS. 1 to 5 illustrate how a first embodiment of a balloon for the balloon catheter of the present invention is formed. A hollow tube 1 is formed of a flexible and resilient elastomeric material 2, in this example a polyurethane. The material 2 is reinforced with braided PET mono-filaments 3, half of which trace out right-handed parallel helixes, and the other half of which trace out left handed parallel helixes. The helixes are crossed at points, but the PET fibres are not bonded to each other at these points. The PET filaments 3 are completely surrounded by the polyurethane. The tube of this example has an outer diameter of 6 mm, an inner diameter of 5.9 mm, and a length of 25 mm. These dimensions may be larger or smaller, depending on the application for the balloon catheter. The PET fibre thickness in this example is about 40 μm, which can readily be completely contained within the wall thickness of about 100 μm for the balloon even where the fibres cross over one anothe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com