Convalent conjugates of biologically-active compounds with amino acids and amino acid derivatives for targeting to physiologically-protected sites
a biologically active compound and conjugate technology, applied in the field of specific targeting of biologically active compounds, can solve the problems of limiting the therapeutic efficacy of such pharmaceutical compounds, preventing the treatment of neurologic diseases, and further limiting the use of even effective neurologic agents, so as to achieve more specificity and effective intracellular concentration of such compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
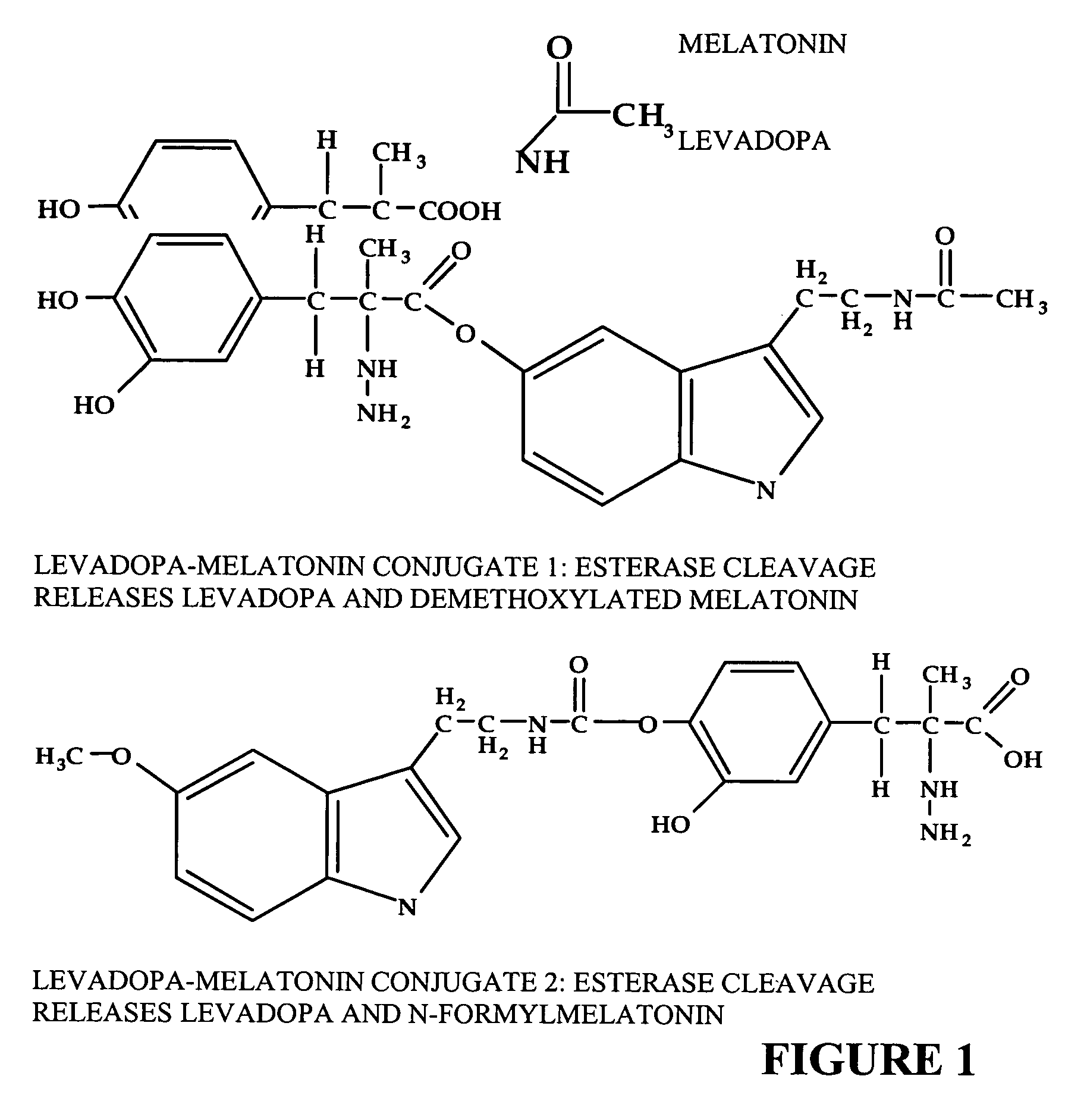
[0123] Conjugates between melatonin and melatonin derivatives with biologically-active compounds were prepared as follows.
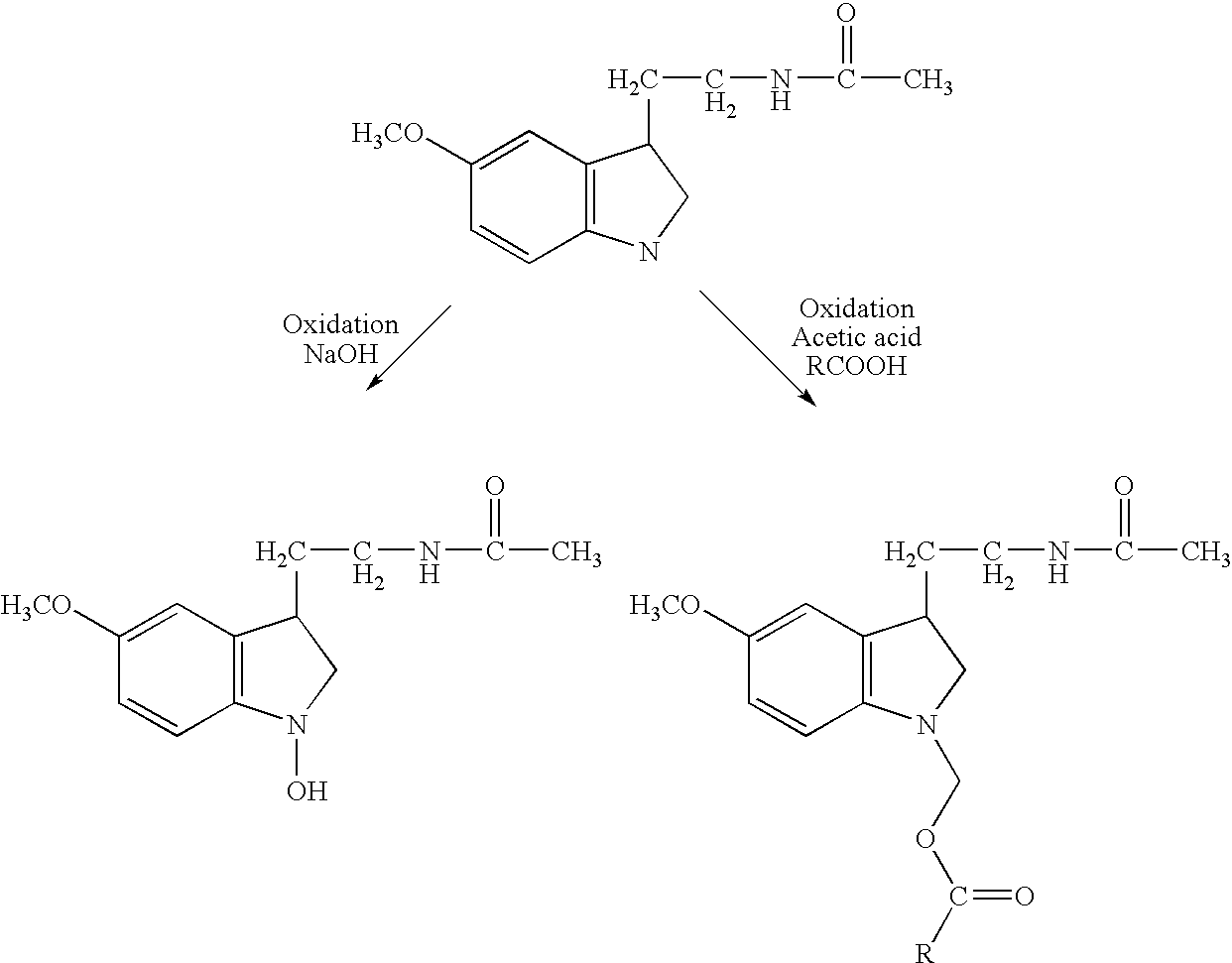
[0124] As a first step, melatonin is converted to modifiable melatonin derivatives, illustrated herein by the indole N—OH and indole N-formoxy ester derivatives.
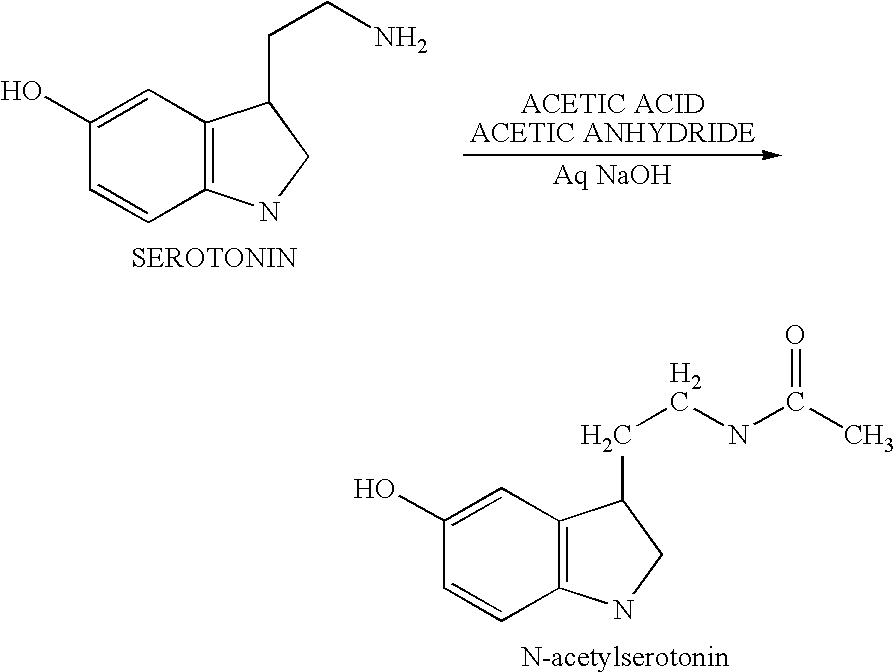
[0125] Alternatively, serotonin is converted to demethoxylated melatonin (N-acetyl serotonin).
[0126] Levadopa conjugates as shown in FIG. 1 can be prepared from either of the indole N melatonin derivatives. In the following synthetic scheme, all hydroxyls and hydrazine protons of levadopa are protected by reaction with trimethyl silyl chloride to form TMS adducts. These adducts are removed after reaction by treatment in dilute acid.
or the ring hydroxylated product:
Synthesis of N-Hydroxy Melatonin
[0127] The synthesis of N-hydroxy melatonin uses conditions described by Bilaski and Ganem (1983, Synthesis, p.537). Briefly, to a 100 mL round-bottomed flask is added 1 g (4.3 mmol) melatonin, 50 mL of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| psychological disorder | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| aqueous-insoluble | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com