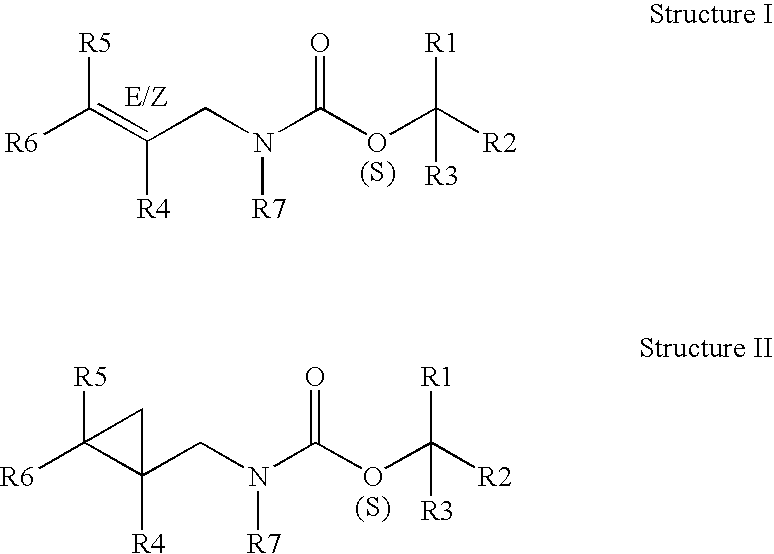
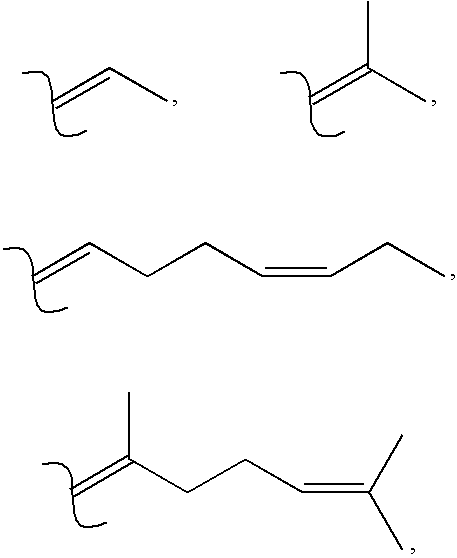
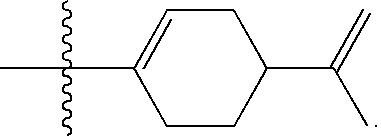
Unsaturated cyclic and acyclic carbamates exhibiting taste and flavor enhancement effect in flavor compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Materials of the Present Invention
[0078] The following reaction sequence was used to prepare the specific compounds described by the NMR data set forth below:
[0079] The amine and triethylamine were dissolved in dichloromethane or ethyl acetate to which the corresponding chloroformate was added in 1.0 to 2.0 equivalents at temperatures ranging from 0° C. to room temperature, most preferably from 5° C. to 15° C. The resulting solution was aged for about 1-3 hours at room temperature.
[0080] The reaction was quenched with aqueous sodium chloride, hydrogen chloride or sodium hydroxide depending upon the need to remove residual acid or amine. The mixture was extracted into ethereal solvent or dichloromethane, washed to neutrality and solvent removed.
[0081] The crude product was purified by distillation or recrystallization depending on the physical properties.
[0082] The carbamates were synthesized according to the general scheme above with the following specific examp...
example ii
[0098] The following reaction sequence was used to make the compound described by the NMR data set forth below:
[0099] Corey's cyclopropanation of compound I, a chemical that is commercially known as Citralva, gives rise to compound II in good yield. The above mentioned transformation proceeds via the trimethylsulfoxium ylide which is generated in-situ from the reaction of trimethylsulfoxium iodide, (CH3)3SOI, and sodium hydride in dimethylsulfoxide, DMSO. Next, metal hydride reduction of compound II affords compound III quantitatively.
Ethyl ([2-methyl-2-(4-methylpent-3-en-1-yl)cyclopropyl]methy)carbamate
[0100] 1-[2-methyl-2-(4-methylpent-3-en-1-yl)cyclopropyl]methanamine (20.0 g, 0.12 mol)in 150 mL of ethyl acetate and ethylchloroformate (17.9 g, 0.165 mol) were charged to a reaction flask. The reaction mixture was then cooled to 0° C. Triethylamine (23.8 g, 0.165 mol) was slowly added while maintaining the reaction temperature at 0° C. Once the feed was complete the reaction mi...
example iii
[0101] The following reaction sequence was used to make the compound described by the NMR data set forth below:
Ethyl (2-[(3Z)-hex-3-en-1-yl]cyclopropyl)methyl)carbamate
[0102] Compound IV is a commercially available starting material (AVAILABLE from Bedoukian Research). The conversion of IV to V is a known transformation, which proceeds via an oxime intermediate. Subsequent transformations to the final product follow a similar synthetic scheme to that of example II 1-(2-[(3Z)-hex-3-en-1-yl]cyclopropyl)methanamine 1 eq 1.4 eq of ethylchloroformate, 1.4 eq of triethylamine, quenched with saturated sodium chloride solution, 70% yield. 1H NMR (500 MHz, CDCl3) δ: −0.12 −0.63 (m, 2H), 0.67-0.74 (m, 1H), 0.81-0.88 & 0.95-0.99 (m, 1H), 0.96 (td, J=7.53, 1.09 Hz, 3H), 1.18-1.29 & 1.44-1.51 (m, 1H), 1.24 (t, J=7.06 Hz, 3H), 1.30-1.39 (m, 1H), 1.98-2.08 (m, 2H), 2.09-2.17 (m, 2H), 3.01-3.05 (m, 1H), 3.11-3.24 (m, 1H), 4.11 (q, J+6.50 Hz, 2H), 4.60-4.77 (br.s, 1H), 5.31-5.47 (m, 2H)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com