6-Monoacetylmorphine derivatives useful in immunoassay
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
specific embodiments
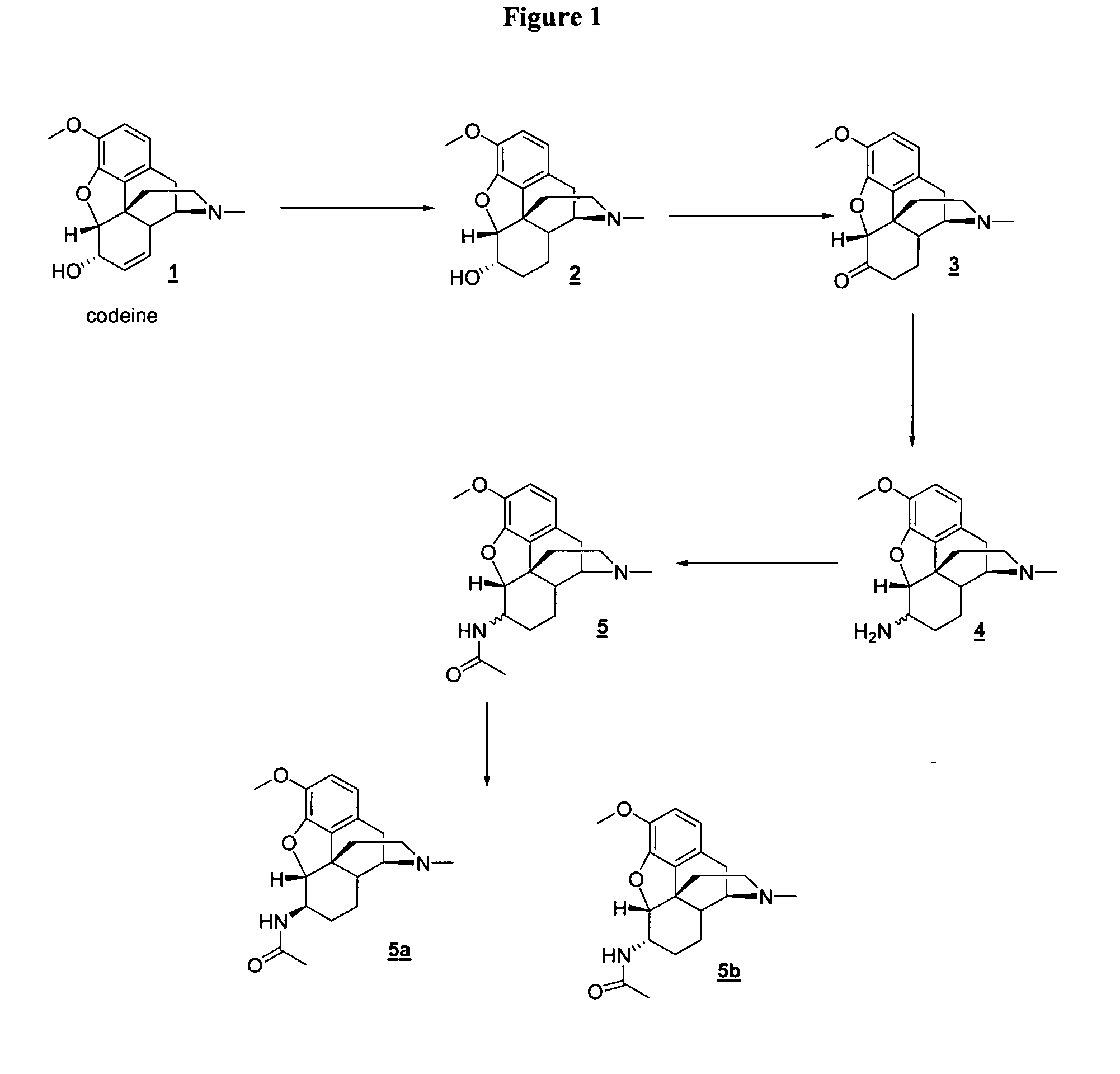
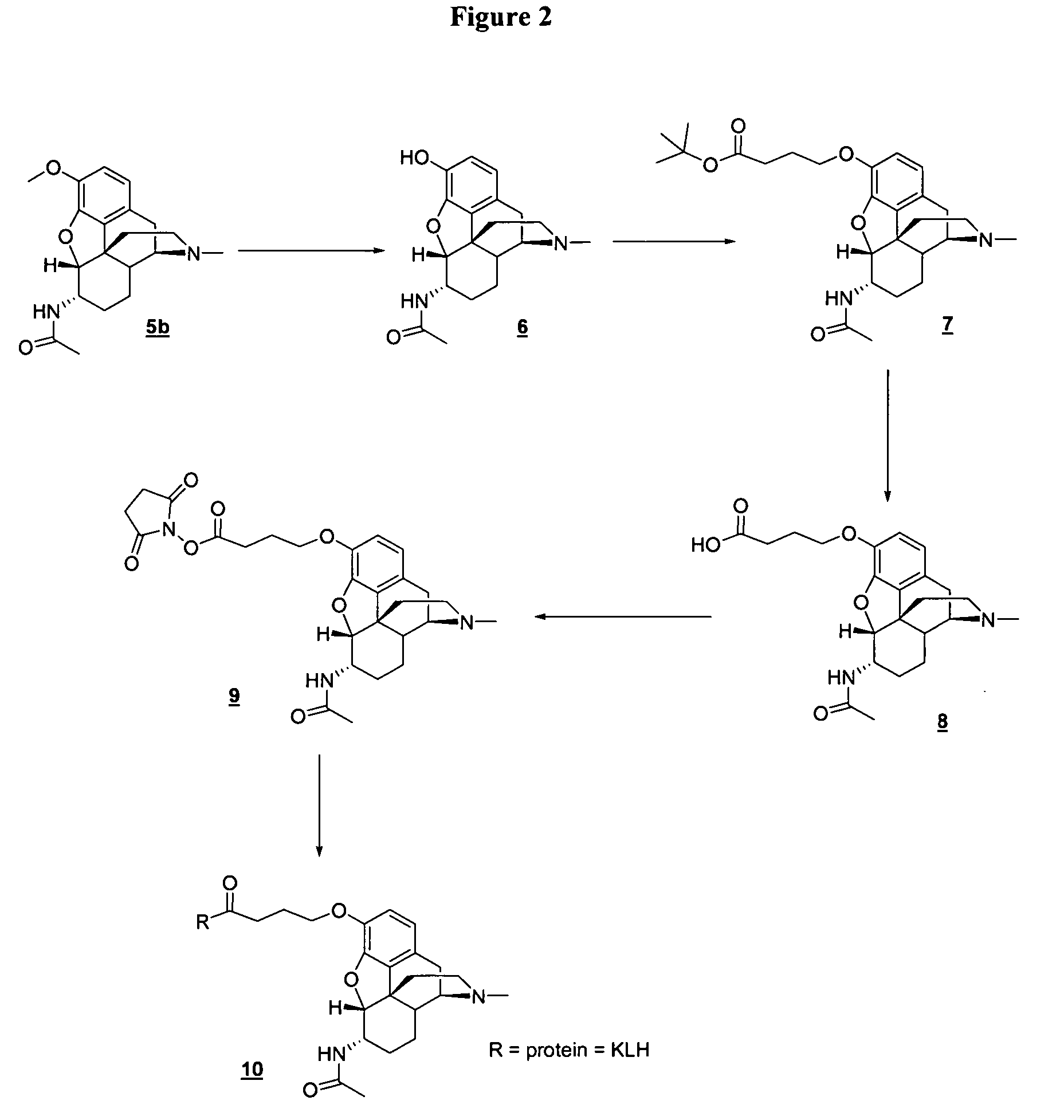
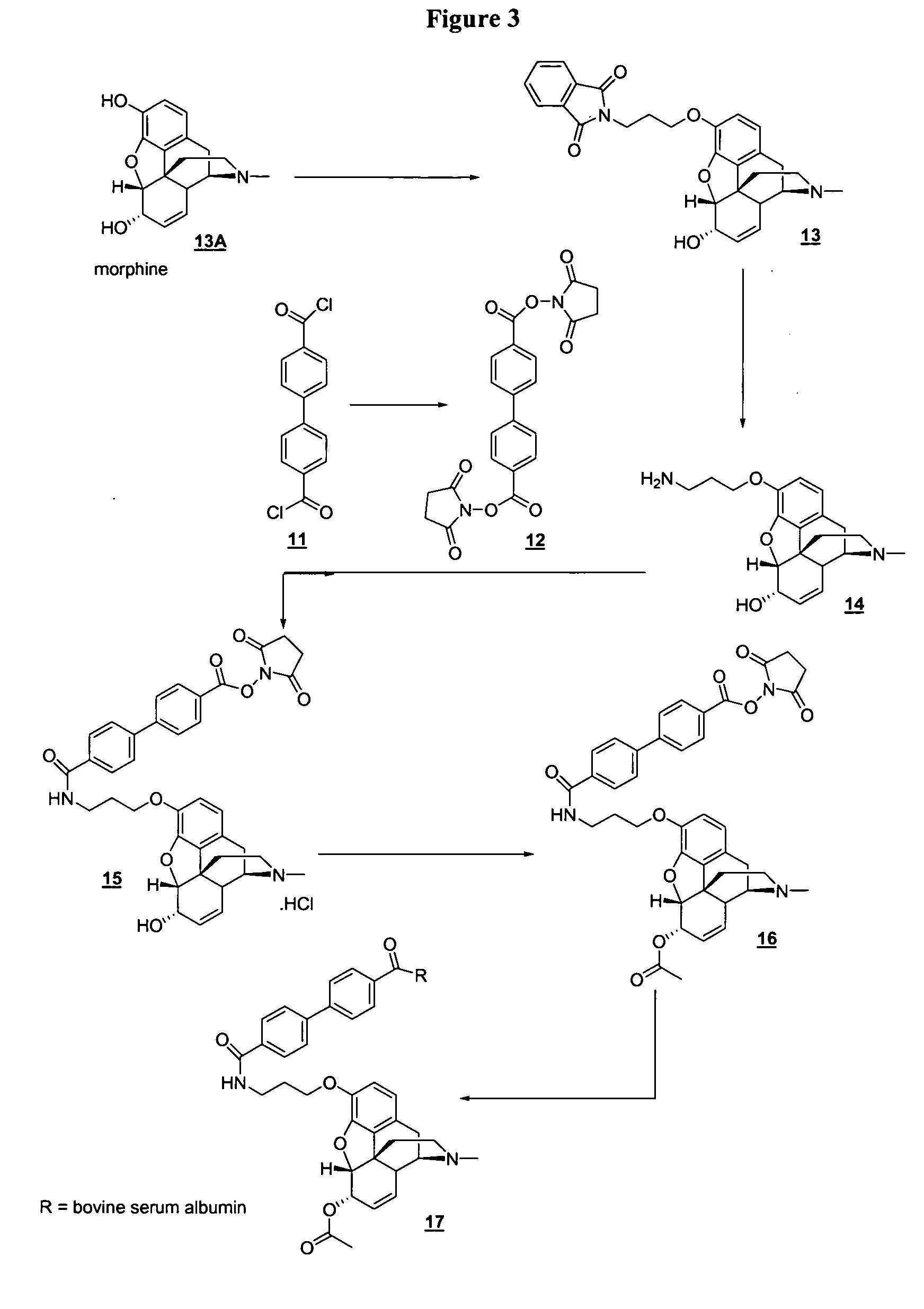
[0054] In the examples that follow, boldface numbers refer to the corresponding structure in the drawings.
example 1
Preparation of Dihydrocodeine (2)
[0055] To 1.2 g (4.0 mmol) of codeine in 40 mL of methanol was added 100 mg of 10% Pd-C. The resulting reaction mixture was hydrogenated under pressure of 50 psi. The solution was filtered through CELITE (Celite Corporation), and the filtrate was concentrated to give 1.12 g (3.7 mmol, 93%) of dihydrocodeinone as a white solid. ES (+) m / z 301.
example 2
Preparation of Dihydrocodeinone (3)
[0056] To a mixture of 1.25 g (11.1 mmol) of potassium-t-butoxide in 50 mL of benzene were added 6.78 g (37 mmol) of benzophenone and 1.12 g (3.7 mmol) of dihydrocodeine, which was allowed to reflux for 2 hours. The reaction mixture turned yellow. The reaction mixture was allowed to cool to room temperature, and 50 mL of 2N HCl was added. The resulting reaction mixture was allowed to stir for 10 minutes. The organic layer was separated and extracted with 3×40 mL of 2N HCl. The organic layer was discarded, and the aqueous layer was again extracted with 3×20 mL of dichloromethane. The aqueous layer was basified with aqueous potassium hydroxide solution to pH 12 and extracted with 5×60 mL of dichloromethane. The organic layers were combined and dried over MgSO4 and filtered. The filtrate was concentrated to give 691 mg (2.3 mmol, 55%) of 3 as a yellow solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com