Biomarker of improved intestinal function
a biomarker and intestinal function technology, applied in the field of intestinal function biomarkers, can solve the problems of not always the case, each suffers from potential bias, and is less sensitive than desired for small changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
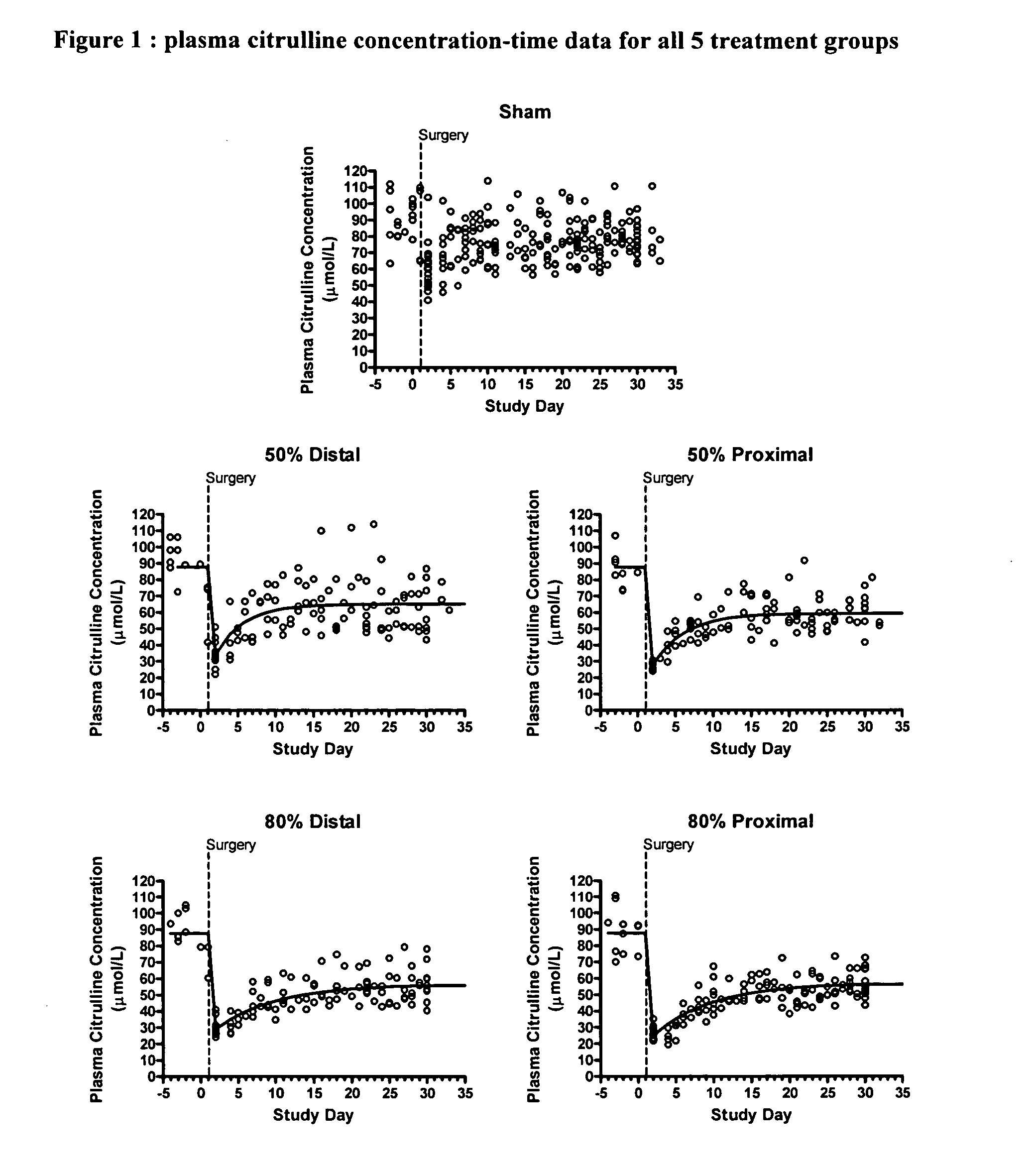
[0019] The intestinal adaptation process has been studied intensively for over 100 years, yet, even today, the tools used to characterize the adaptive process are complicated and potentially biased. One well-established model system used to study intestinal adaptation is that of small bowel resection in rats. The time course of the adaptive process in rats is well characterized in this model, with complete adaptation occurring within 30 days following intestinal resection (Dowling and Booth, 1967; Hanson et al., 1977). These estimates have been obtained using invasive techniques that require multiple animals to be sacrificed at each time point, denying the opportunity to observe the progression of an individual animal. Furthermore, these invasive techniques are inappropriate for a clinical study.
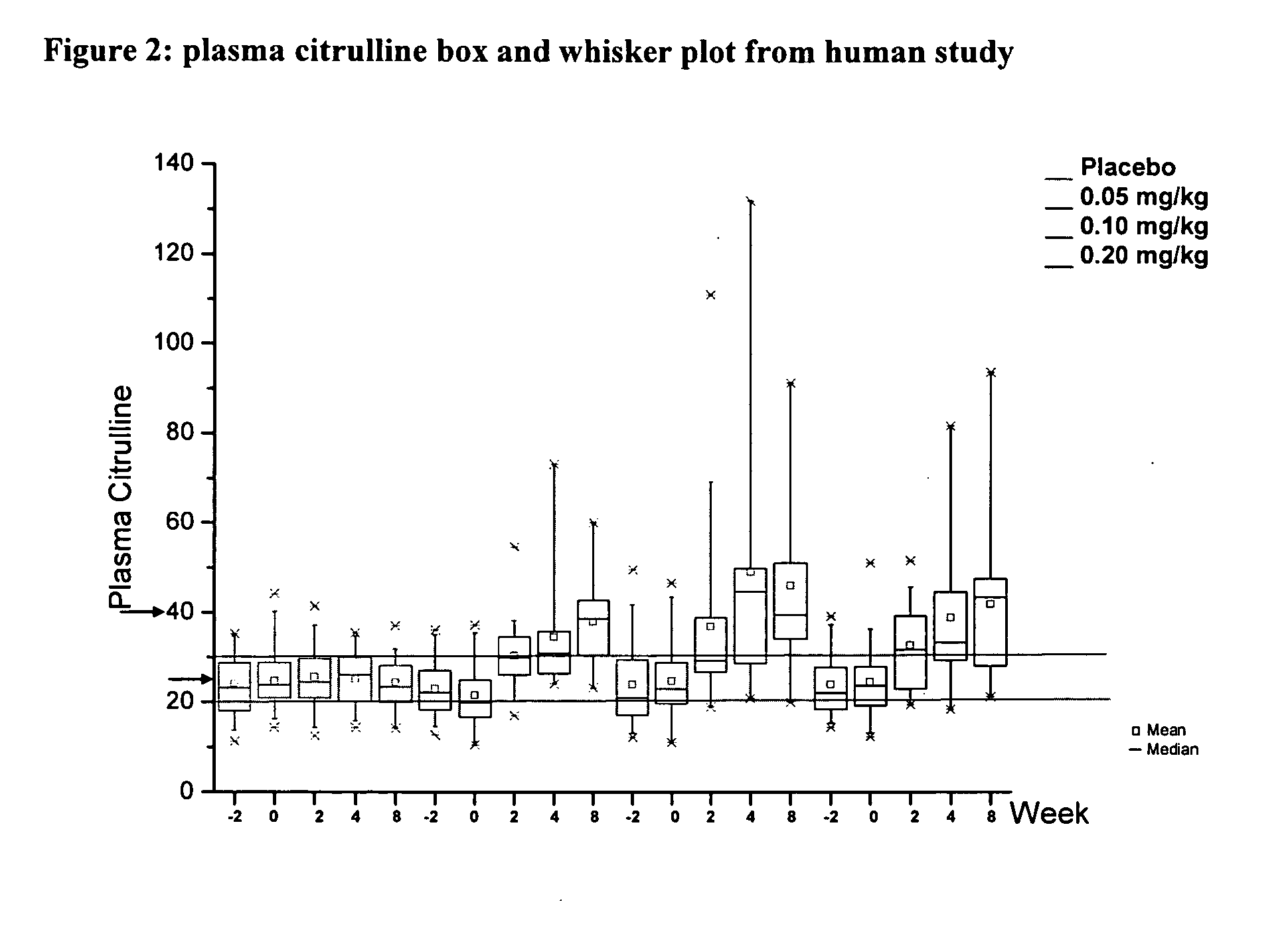
[0020] For the first time, a pharmacodynamic model of plasma citrulline as a marker for intestinal function and adaptation has been developed. The exponential growth model was fit to plasma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com