Fluoroquinolone compositions
a technology of fluoroquinolone and composition, applied in the field of pharmaceutical compositions, can solve the problems of preventing the use of nalidixic acid in other types of infections, unable to meet the needs of patients, and difficult to swallow solid oral pharmaceutical compositions, etc., and achieve the effect of convenient administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
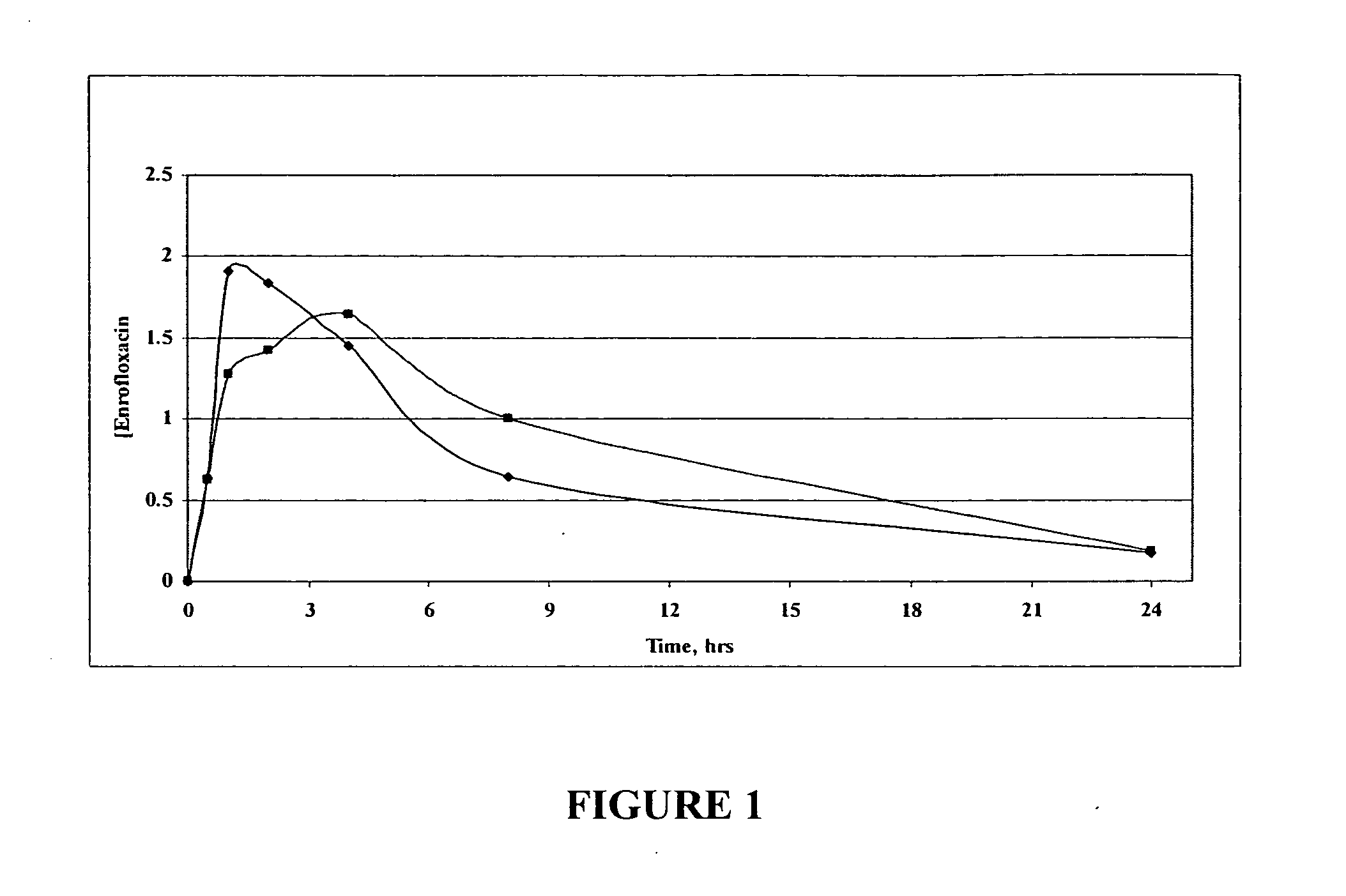
Oral Liquid Enrofloxacin Composition—Zinc Cation and Strawberry Flavored
[0294] 2.3 gr of enrofloxacin and 0.7024 gr of zinc acetate were mixed well with 15 mL of de-ionized water in a 100 ml volumetric flask. To the resulting composition was added 60 mL of glycerol with mixing to provide a homogeneous solution. To this solution was added 5 mL of strawberry flavor and the resulting solution mixed well. The volume of the solution was made up to 100 mL with glycerol and mixed well to provide a liquid oral enrofloxacin formulation. The concentration of enrofloxacin in the formulation is 23 mg / mL.
[0295] A pharmaceutical composition similar to that described above can be prepared except that the flavoring is not included in the composition and the pharmaceutical composition is sterile filtered to provide an injectable pharmaceutical composition.
example 2
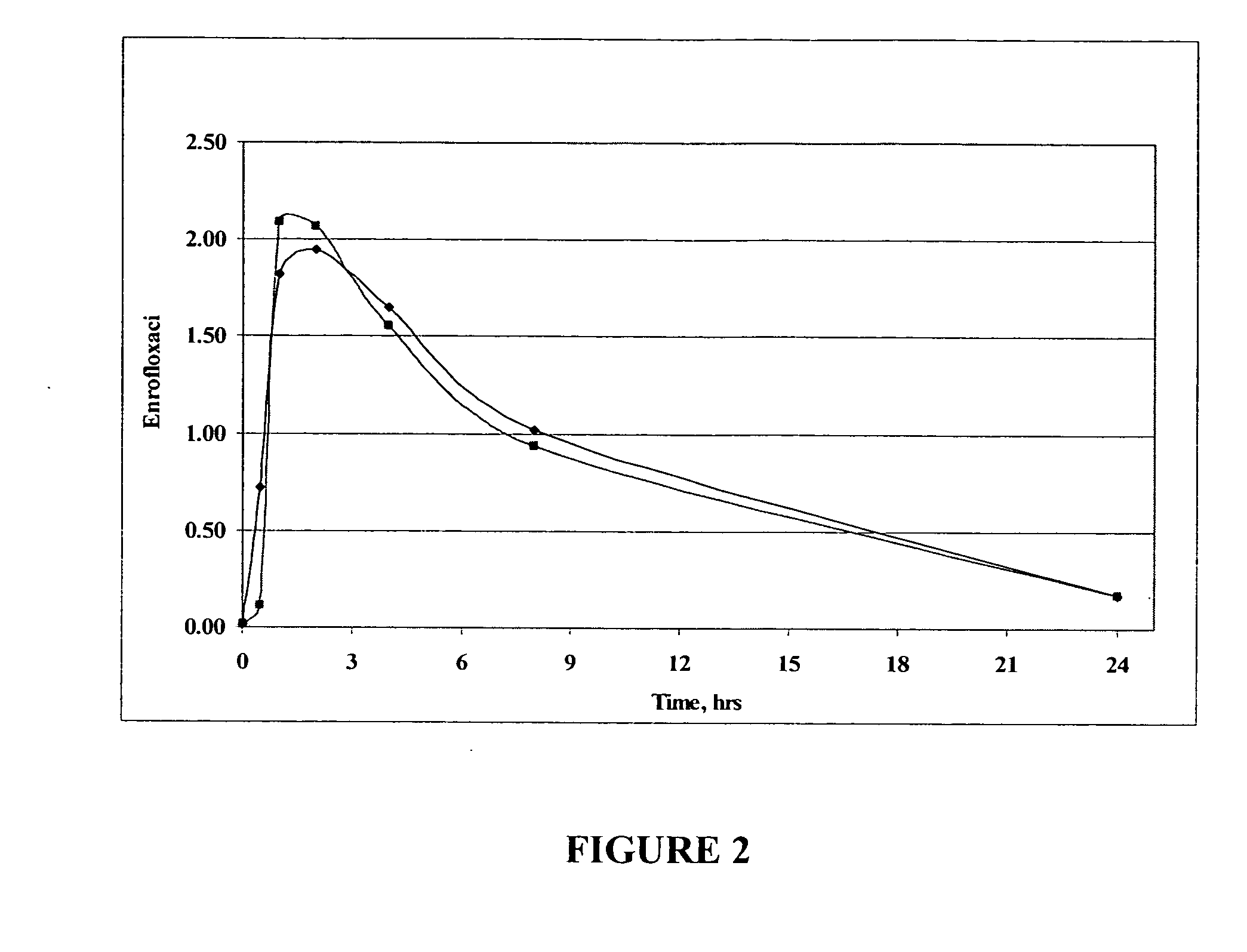
Oral Liquid Enrofloxacin Composition—Magnesium Cation and Strawberry Flavored
[0296] 575 mg of enrofloxacin and 114 mg of magnesium acetate (0.8 mL of 1 M aqueous solution, commercially available from Sigma-Aldrich of Milwaukee, Wis.) were mixed well with 3.75 mL of de-ionized water in a 25 mL volumetric flask. To the resulting composition was added 15 mL of glycerol with mixing to provide a homogeneous solution. To the resulting solution was added 1.25 mL of strawberry flavor with mixing. The volume of the solution was made up to 25 mL with glycerol and mixed well to provide a liquid oral enrofloxacin formulation. The concentration of enrofloxacin in the formulation is 23 mg / mL.
[0297] A pharmaceutical composition similar to that described above can be prepared except that the flavoring is not included in the composition and the pharmaceutical composition is sterile filtered to provide an injectable pharmaceutical composition.
example 3
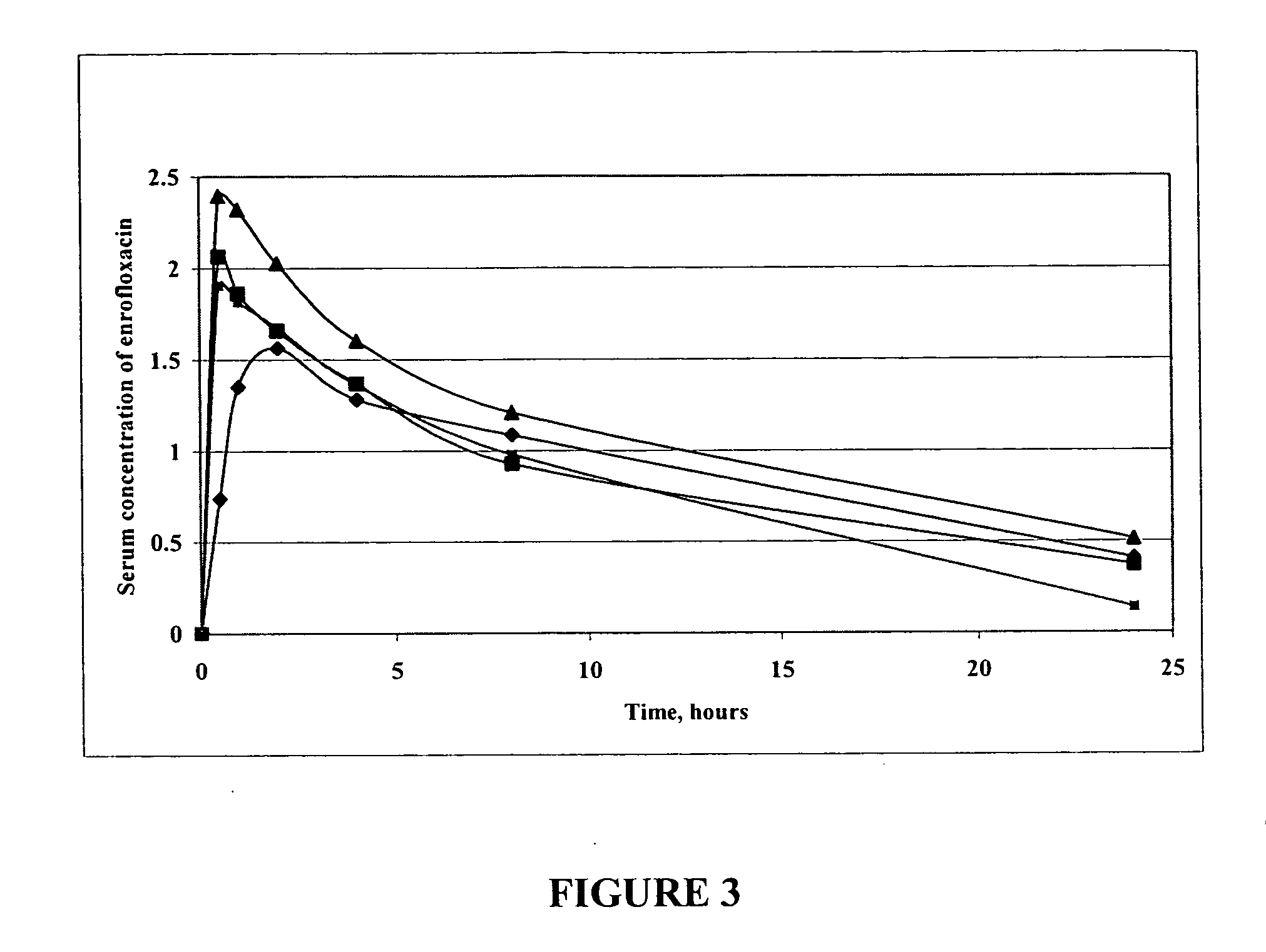
Oral Liquid Enrofloxacin Composition—Magnesium Cation and Mint Flavored
[0298] 575 mg of enrofloxacin and 114 mg of magnesium acetate (0.8 mL of 1 M aqueous solution, commercially available from Sigma-Aldrich of Milwaukee, Wis.) were mixed well with 3.75 mL of de-ionized water in a 25 ml volumetric flask. To the resulting composition was added 15 mL of glycerol with mixing to provide a homogeneous solution. To the resulting solution was added 1.25 mL of mint oil with mixing. The volume of the solution was made up to 25 mL with glycerol and mixed well to provide a liquid oral enrofloxacin formulation. The concentration of enrofloxacin in the formulation is 23 mg / mL.
[0299] A pharmaceutical composition similar to that described above can be prepared except that the flavoring is not included in the composition and the pharmaceutical composition is sterile filtered to provide an injectable pharmaceutical composition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com