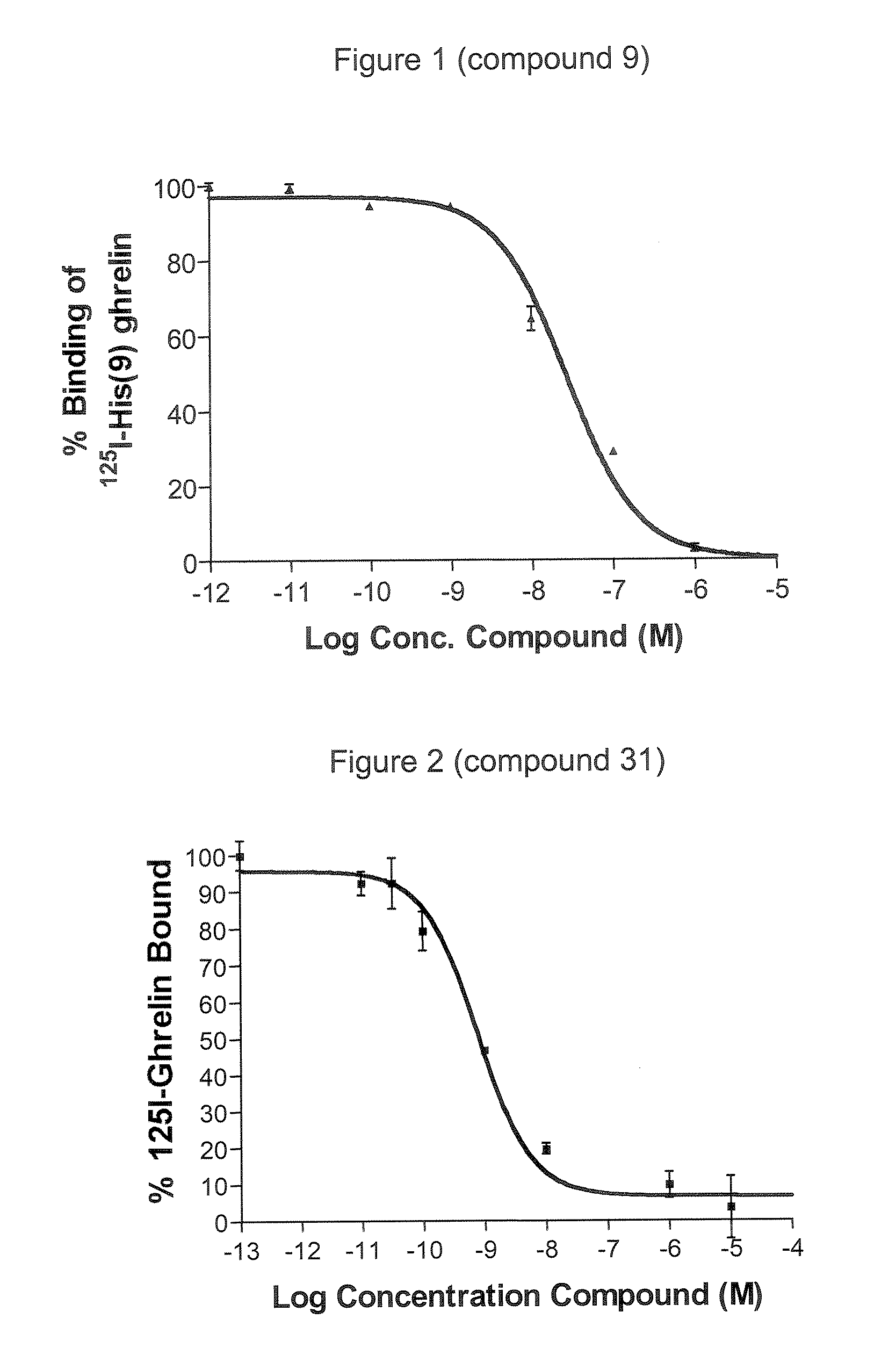
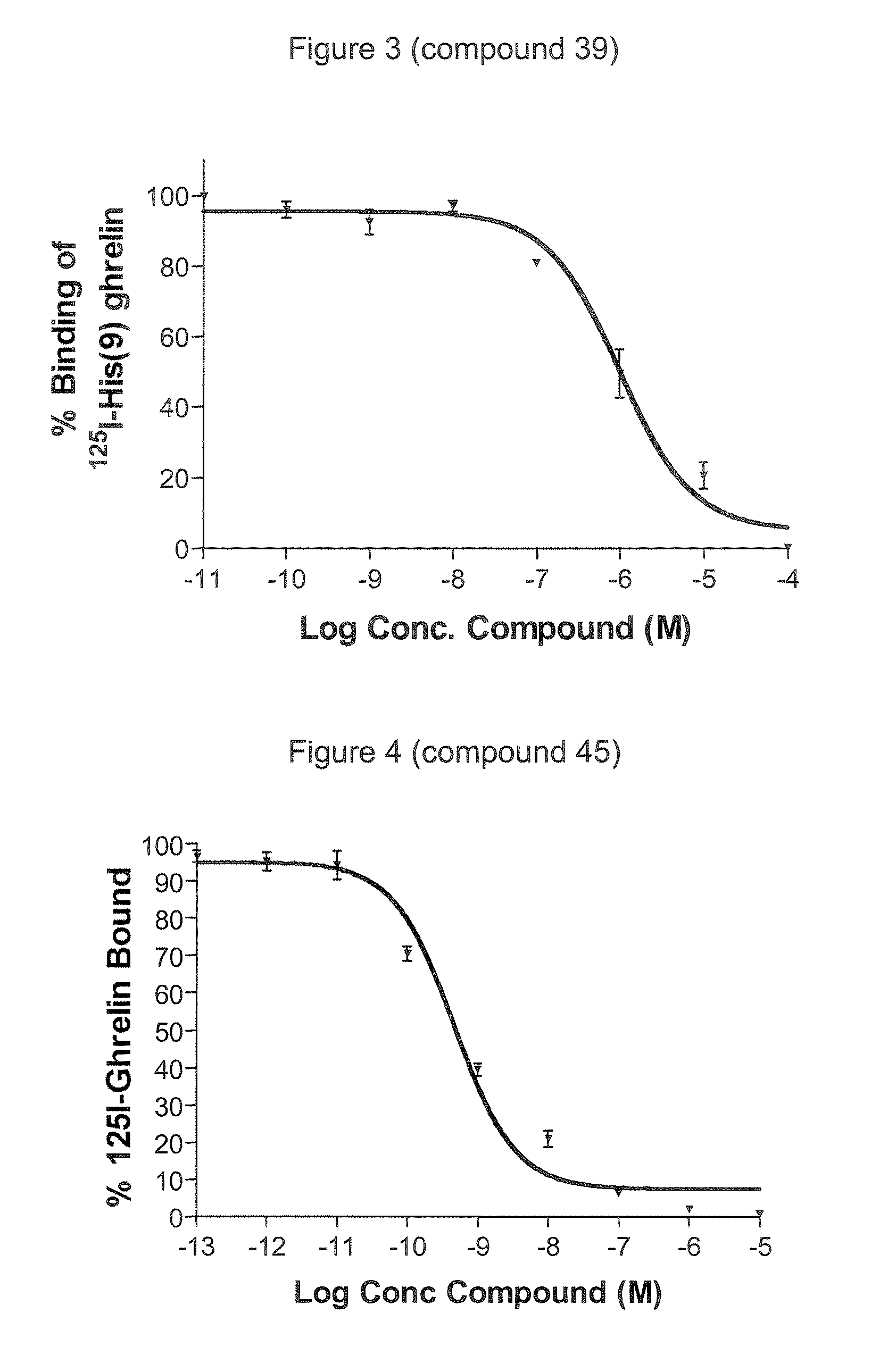
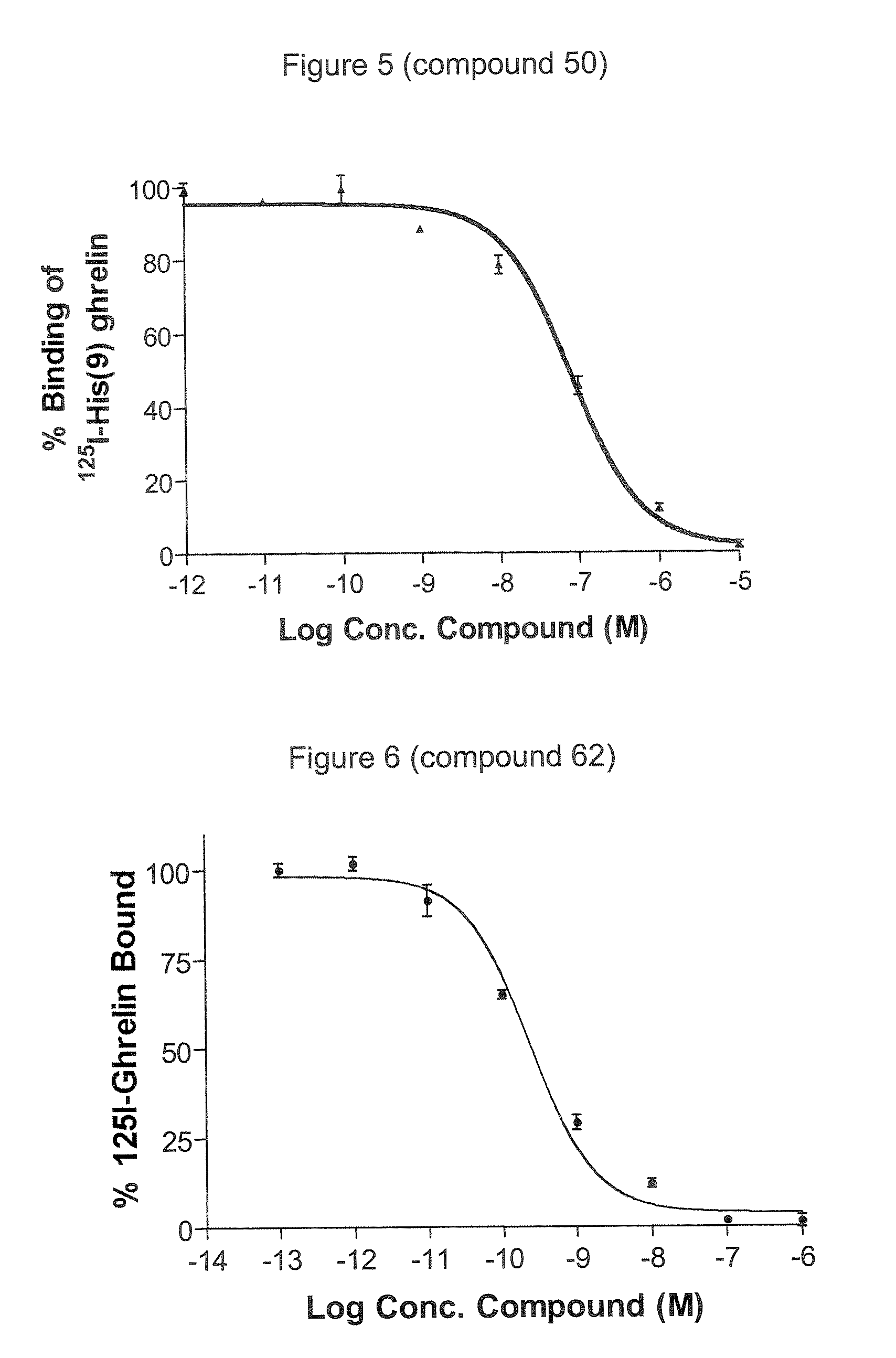
Novel triazole derivatives as ghrelin analogue ligands of growth hormone secretagogue receptors
a growth hormone secretagogue receptor and triazole technology, applied in the field of new triazole derivatives, can solve the problems of not providing any biological activity or medicinal use, not naming specific compounds, and failing to describe and demonstrate inhibitory and/or antagonistic activity of claimed compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(R)—N-(1-(5-(2-(1H-indol-3-yl)ethyl)-4-(2,4-dimethoxybenzyl)-4H-1,2,4-triazol-3-yl)-2-(1H-indol-3-yl)ethyl)-2-amino-2-methylpropanamide (Compound 1)
[0404] Compound 1 was obtained from Boc-(D)-Trp (10 mmoles), (2,4-dimethoxyphenyl)-methanamine, 3-(1H-indol-3-yl)propane hydrazide and Boc-2-amino-2-methylpropanoic acid according to the general synthetic schemes with a total yield after purification by HPLC of 35%.
[0405]1H NMR (400 MHz, 300° K, DMSO-d6):
[0406]δ 1.32 (3H, s, CH3 Aib), 1.36 (3H, s, CH3 Aib), 2.93 (2H, m, CH2—CH2-indole), 2.97 (2H, m, CH2—CH2-indole), 3.31 (1H, dd, J=14.5, J=6.1, 1H C2 βTrp), 3.38 (1H, dd, J=14.5, J=9.1, 1H C2 βTrp), 3.66 (3H, s, o-OCH3), 3.72 (3H, s, p-OCH3), 4.93 (1H, d, J=16.9, 1H C2 o,p-dimethoxybenzyl), 5.10 (1H, d, J=16.9, 1H C2 o,p-dimethoxybenzyl), 5.23 (1H, m, CαH Trp), 6.31 (1H, dd, J=8.5, J=1.7, H5 o,p-dimethoxybenzyl), 6.45 (1H, d, J=8.5, H6 o,p-dimethoxybenzyl), 6.59 (1H, d, J=1.7, H3 o,p-dimethoxybenzyl), 6.88 (1H, t, J=7.5, H5 Trp), 6.94 ...
example 2
(R)—N-(1-(5-benzyl-4-(naphthalen-1-ylmethyl)-4H-1,2,4-triazol-3-yl)-2-(1H-indol-3-yl)ethyl)-2-amino-2-methylpropanamide (Compound 4)
[0410] Compound 4 was obtained from Boc-(D)-Trp (10 mmoles), naphthalen-1-yl-methanamine, 2-phenylacetohydrazide and Boc-2-amino-2-methylpropanoic acid according to the general synthetic schemes with a total yield after purification by HPLC of 42%.
[0411]1H NMR (300 MHz, DMSO-d6, 300° K):
[0412]δ(ppm) 1.18 (3H, s, CH3 Aib), 1.24 (3H, s, CH3 Aib), 3.17 (1H, dd, J=14 Hz and 5 Hz, CH2 βTrp), 3.36 (1H, dd, J=14 and 9 Hz, CH2 βTrp), 4.05 (2H, m, CH2-benzyl), 4.90 (1H, m, CH αTrp), 5.65 (1H, d, J=18 Hz, CH2-naphtyl), 5.81 (1H, d, J=18 Hz, CH2-naphtyl), 6.12 (1H, d, Jo=7 Hz, H2 naphtyl), 6.38 (1H, t, Jo=7 Hz, H5 Trp), 6.47 (1H, d, Jo=8 Hz, H4 Trp), 6.85 (1H, t, Jo=8 Hz, H6 Trp), 7.03 (1H, d, Jm=2 Hz, H2 Trp), 7.05-7.12 (5H, m, CHar benzyl), 7.15 (1H, d, Jo=8 Hz, H7 Trp), 7.19 (1H, d, Jo=8 Hz, H3 naphtyl), 7.58 (2H, m, H6 and H7 naphtyl), 7.81 (1H, d, Jo=8 Hz,...
example 3
(R)—N-(1-(5-(2-(1H-indol-3-yl)ethyl)-4-(naphthalen-1-ylmethyl)-4H-1,2,4-triazol-3-yl)-2-(1H-indol-3-yl)ethyl)-2-amino-2-methylpropanamide (Compound 5)
[0416] Compound 5 was obtained from Boc-(D)-Trp (10 mmoles), naphthalen-1-yl-methanamine, 3-(1H-indol-3-yl)propane hydrazide and Boc-2-amino-2-methylpropanoic acid according to the general synthetic schemes with a total yield after purification by HPLC of 33%.
[0417]1H NMR (400 MHz, DMSO-d6, 300° K):
[0418]δ(ppm) 1.25 (3H, s, CH3 Aib), 1.28 (3H, s, CH3 Aib), 2.93 (2H, m, CH2—CH2-indole), 3.01 (2H, m, CH2—CH2-indole), 3.30 (1H, dd, 3J=14.3 and 5.8 Hz, CH2 βTrp), 3.40 (1H, dd, 3J=14.3 and 8.8 Hz, CH2 βTrp), 5.03 (1H, m, CH αTrp), 5.62 (1H, d, J=18.0 Hz, CH2-naphtyl), 5.76 (1H, J=18.0 Hz, CH2-naphtyl), 3.36 (1H, d, Jo=7.2 Hz, H2 naphtyl), 6.51 (1H, t, Jo=7.4 Hz, H5 Trp), 6.72 (1H, d, Jo=7.9 Hz, H4 Trp), 6.76 (1H, t, Jo=7.5 Hz, H5 indole), 6.92 (1H, t, Jo=7.5 Hz, H6 Trp), 7.0 (1H, t, Jo=7.5 Hz, H6 indole), 7.02 (1H, d, J=2.0 Hz, H2 indole...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| v/v | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com