Three-dimensional optical memory
a three-dimensional optical memory and optical data technology, applied in the field of three-dimensional optical data storage and retrieval system, can solve the problems of inability to use commercially and high maintenance cost of such a memory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
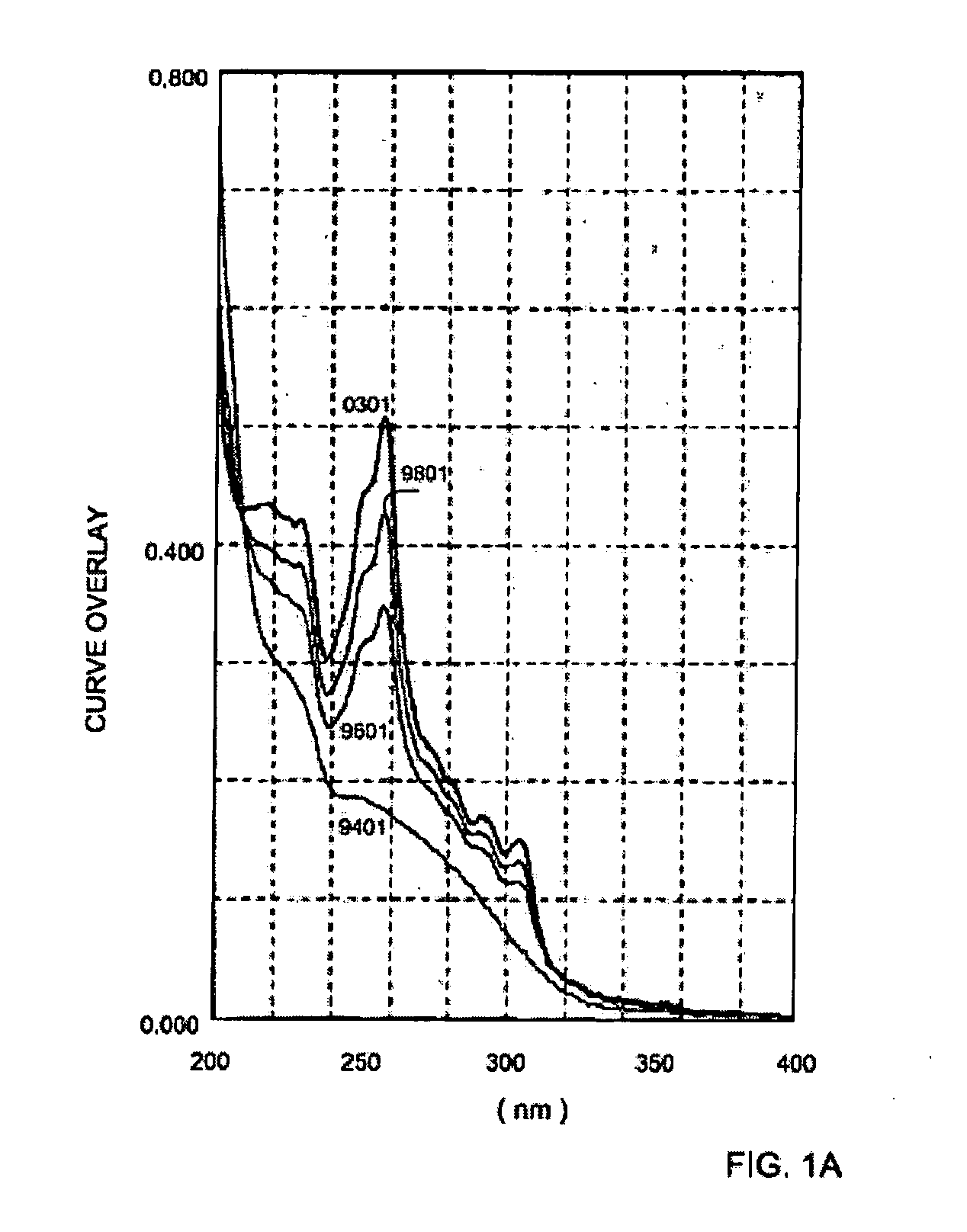
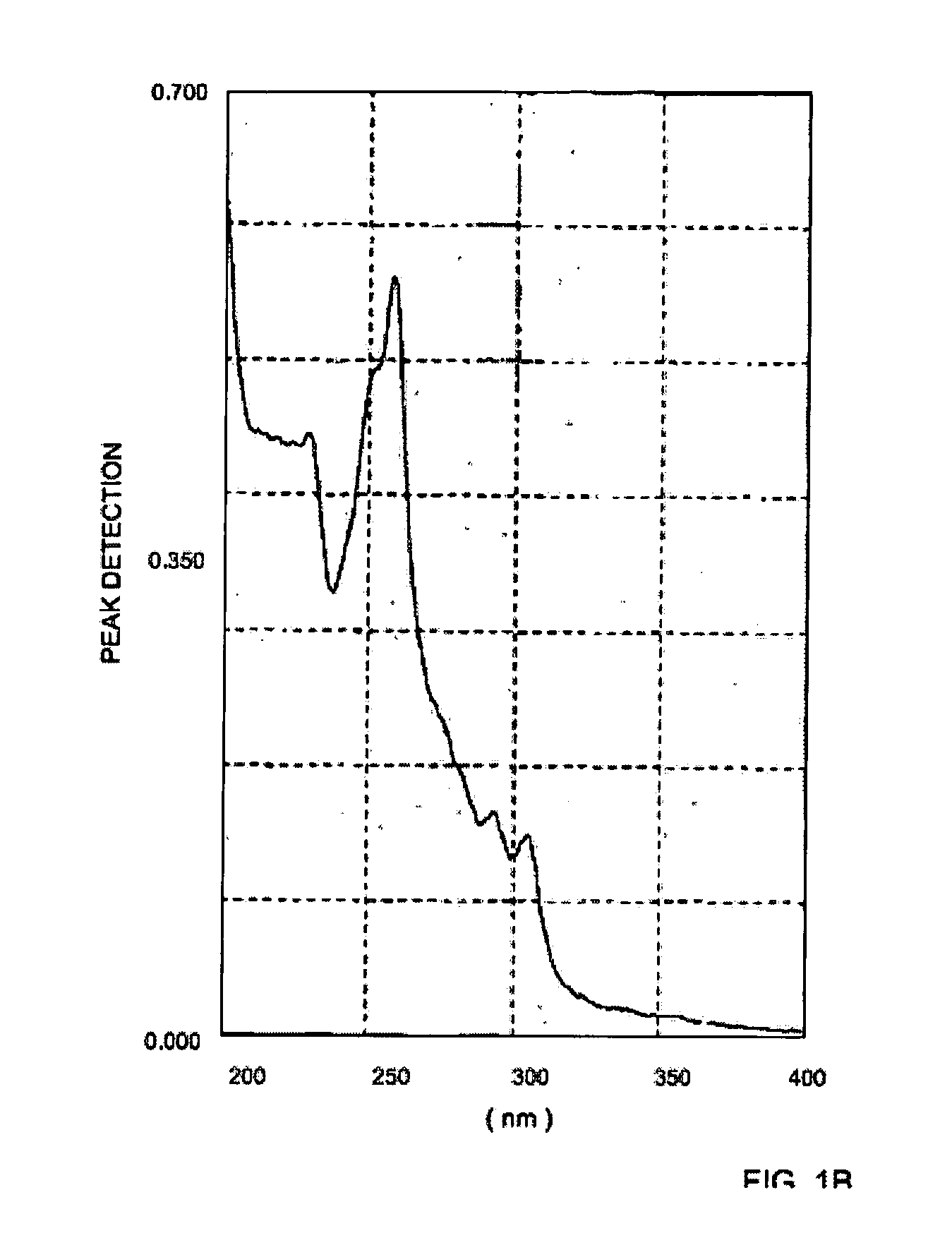
[0045] Pure trans-4-bromostilbene diethylacetate was dissolved in acetonitrile and irradiated with a mercury lamp having a Hg filter. The U.V. spectrum displayed in FIG. 1A illustrates the spectrum of the pure trans isomer (designated 9401). The cis isomer leads to a photoproduct that has a strong absorption at 254 nm, and thus also shown are the resulting spectrum of the formed mixture of compounds after 5 minutes of irradiation (designated 9601), the resulting spectrum of the formed mixture of compounds after 8 minutes of irradiation (designated 9801) and the resulting spectrum of the formed mixture of compounds after 15 minutes of irradiation (designated 0301). FIG. 1B illustrates the spectrum of the residue after 18 hrs of irradiation.
example 2
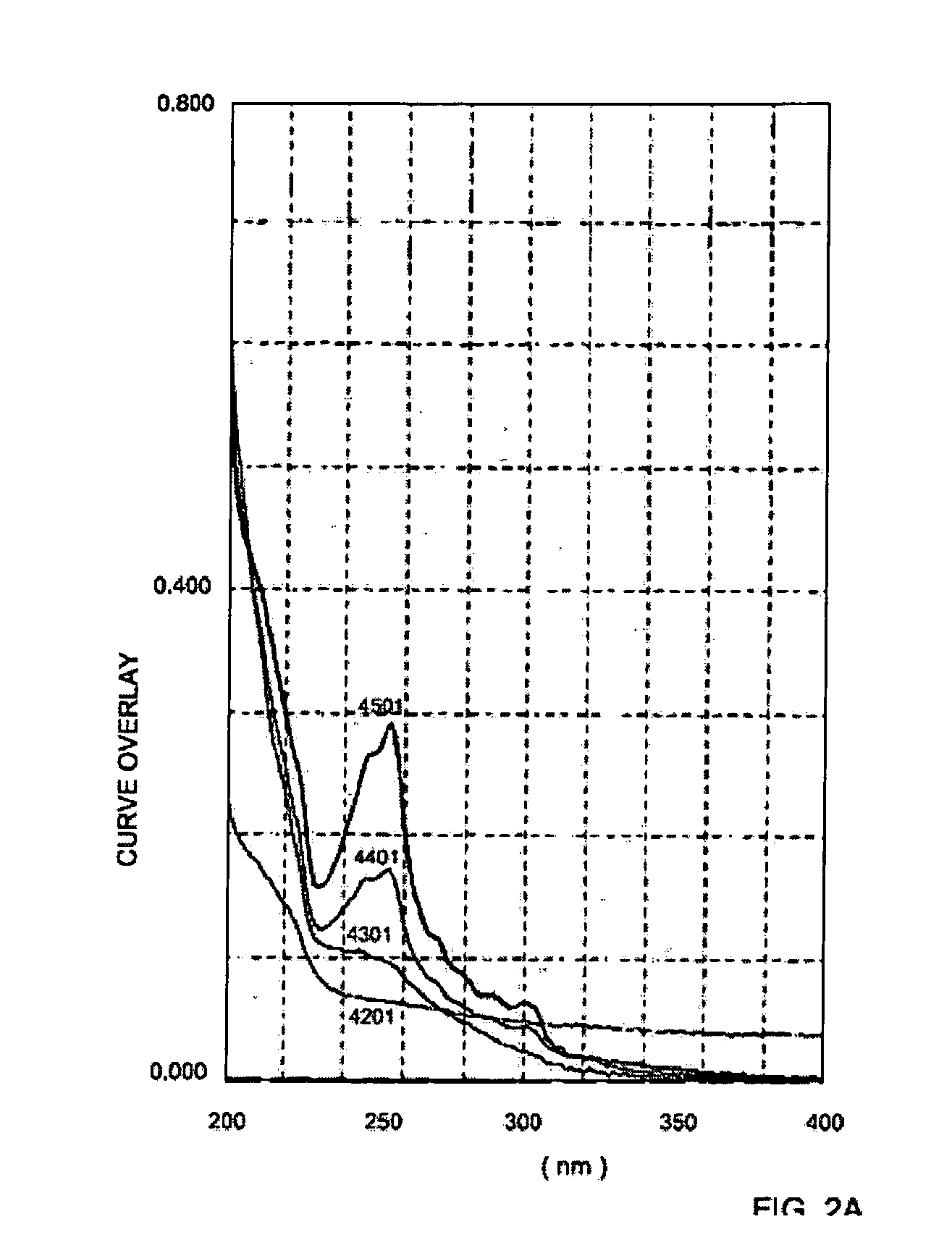
[0046] Pure trans-stilbene dipropanol was dissolved in acetonitrile and irradiated with a mercury lamp having a Hg filter. The U.V. spectrum displayed in FIG. 2A illustrates the spectrum of the pure trans isomer (designated 4301), the resulting spectrum of the formed mixture of trans and cis isomers (having a strong absorption at 254 nm) after 2 minutes of irradiation (designated 4401), the resulting spectrum of the formed mixture of trans and cis isomers after 6 minutes of irradiation (designated 4501). The spectrum of the acetonitrlie is designated 4201. FIG. 2B illustrates the spectrum of the cis-stilbene dipropanol as in FIG. 2A, however after the abstraction of the acetonitrile spectrum.
example 3
[0047] trans-stilbene diethylacetate was dissolved in acetonitrile and irradiated with a mercury lamp having a Hg filter. The displayed U.V. spectrum (FIG. 3) illustrates the spectrum of the pure trans isomer (designated 5301), and the resulting spectrum of the formed residue (with the photoproduct arising from the cis-isomer having a strong absorption at 254 nm) after 22 hrs of irradiation (designated 5501)
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| focus size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com