Wound dressings, apparatus, and methods for controlling severe, life-threatening bleeding
a technology of wound dressings and bandages, applied in the field of advanced hemorrhage control bandages, can solve the problems of no low cost wound dressings that address or any wound dressings, bleeding can be fatal in ballistic injuries, severe arterial lacerations, etc., and achieve the effect of low cost and low cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0073] Table 1 provides a list of the main chitosan materials acquired for hemorrhage control testing. With the exception of the Gelfoam™+thrombin, and Surgicel™ controls for swine spleen experiments and the Johnson and Johnson 4×4 gauze control for use in swine aortic perforations, the dressing materials were all chitosan-based.
[0074] Aqueous solutions (2.00% w / w) were prepared in clean, sterile, 1 liter Pyrex flasks from Ametek UF water and dry chitosan. In the case of the Carbomer, Primex and Genis chitosan materials, 1.0% or 2.0% w / w of glacial acetic acid (Aldrich 99.99%) was added to the aqueous mixtures. Dissolution was achieved by shaking of the flask at 40° C. for 12 to 48 hours. The solutions were degassed by application of vacuum at 500 mTorr at room temperature immediately prior to freezing.
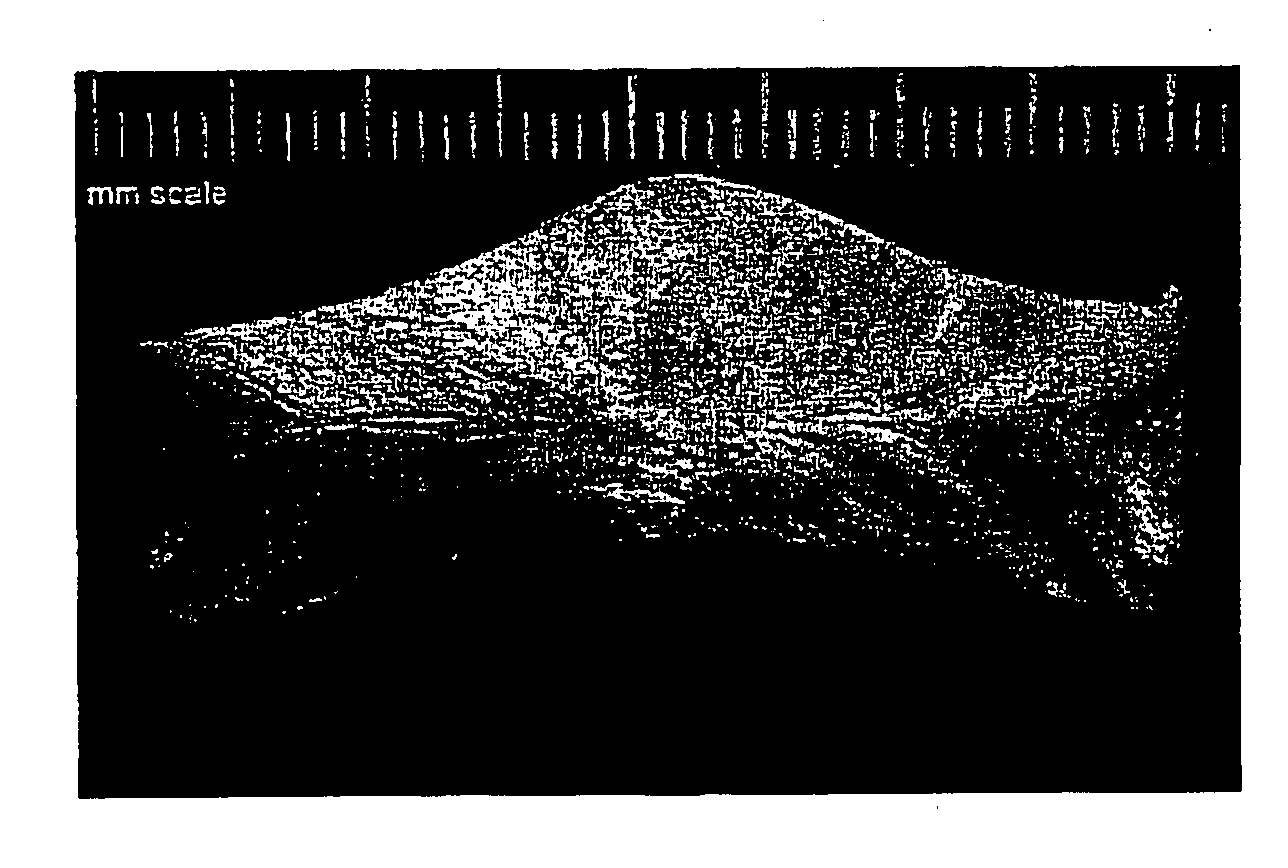
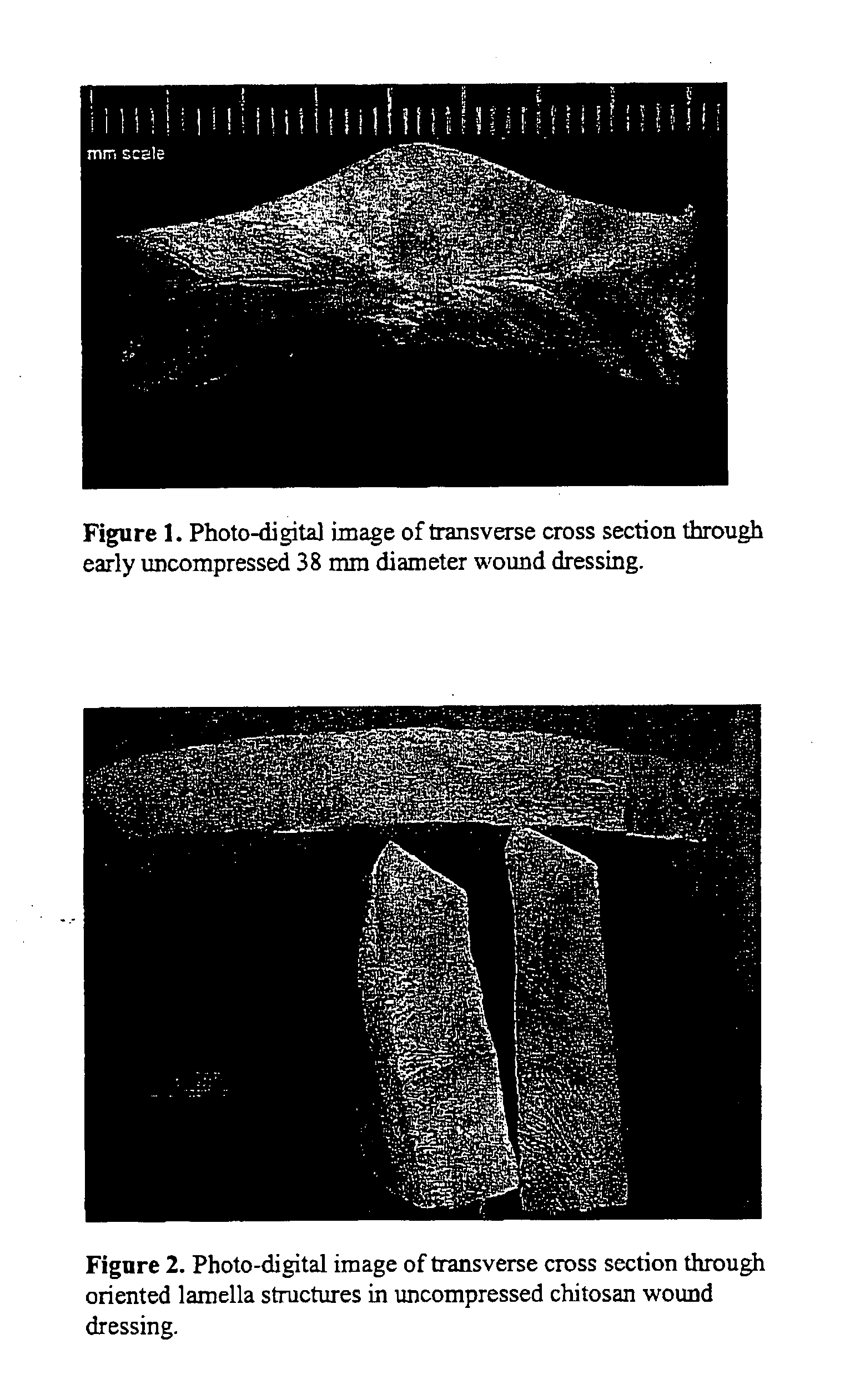
[0075] Wound dressings were prepared from the 2% aqueous solutions of chitosan that were poured into Teflon™ coated aluminum or polystyrene molds to at least 1.5 cm deep and frozen ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| systolic blood pressure | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| pore diameters | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com