Method, code, and system for assaying joint deformity
a joint deformity and code technology, applied in the direction of material analysis using wave/particle radiation, instruments, diagnostic recording/measuring, etc., can solve the problems of various limitations in the ability of methods to pinpoint and accurately quantitate joints
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
A. EMBODIMENT 1
Measuring JWS
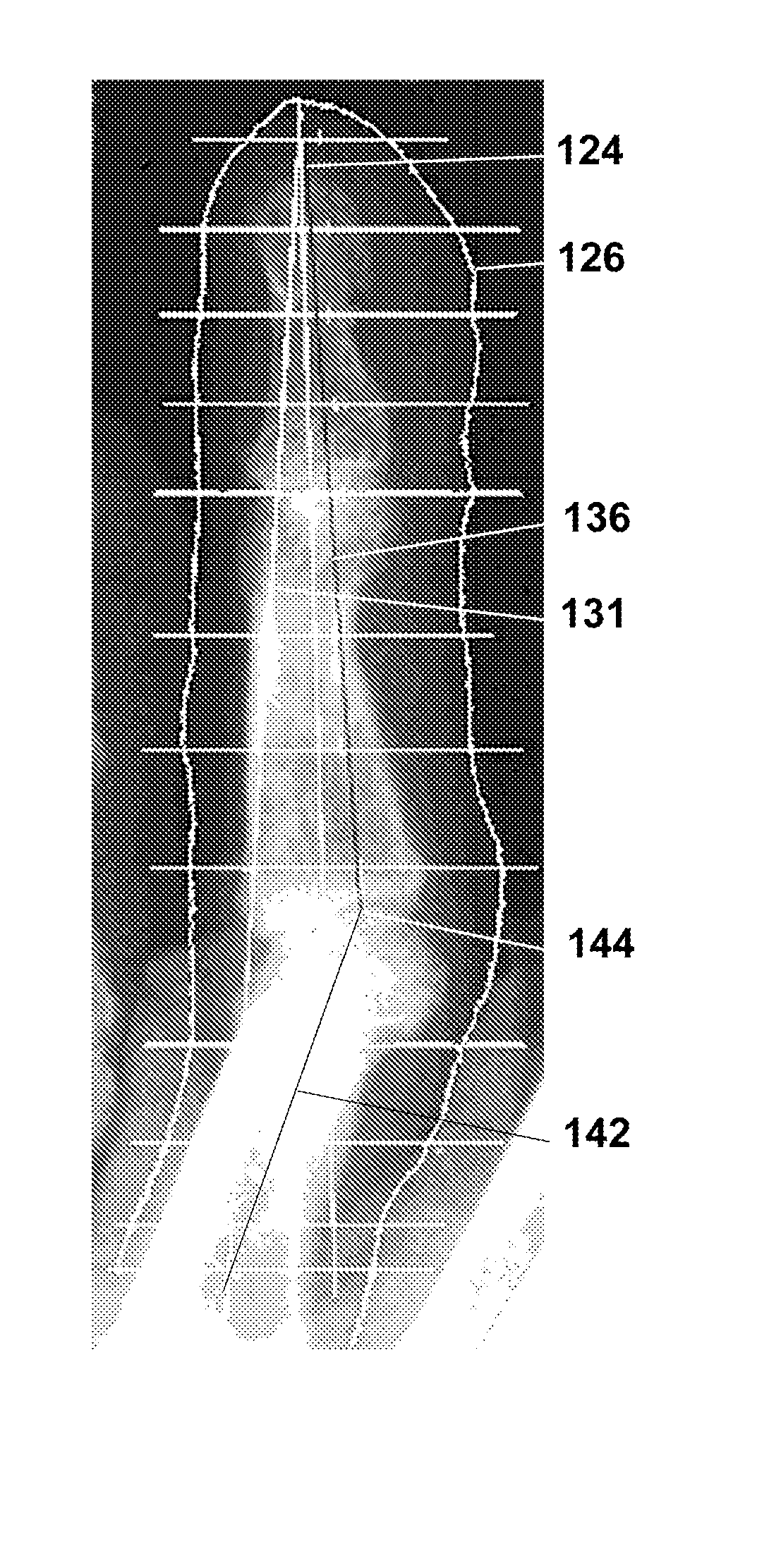
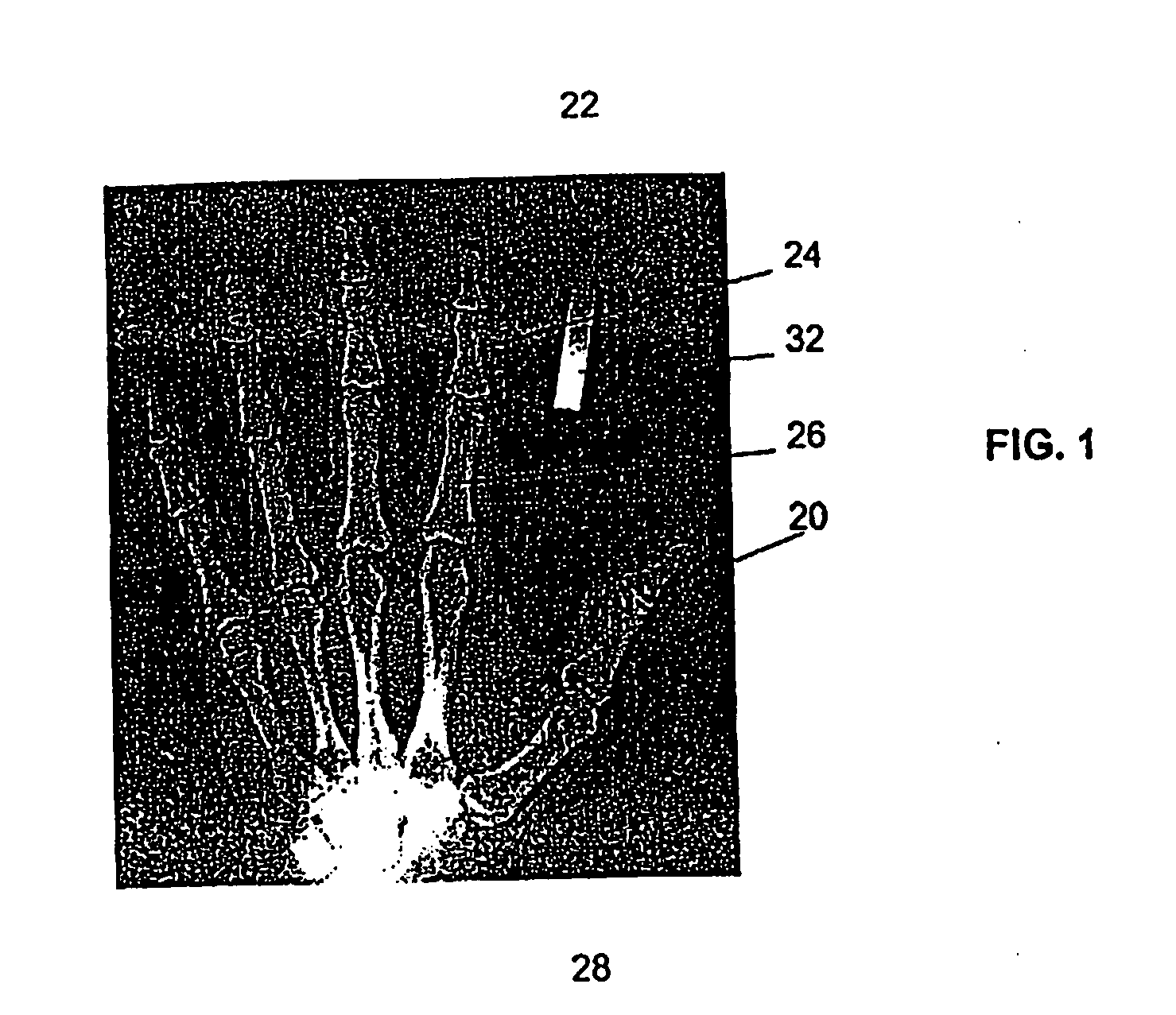
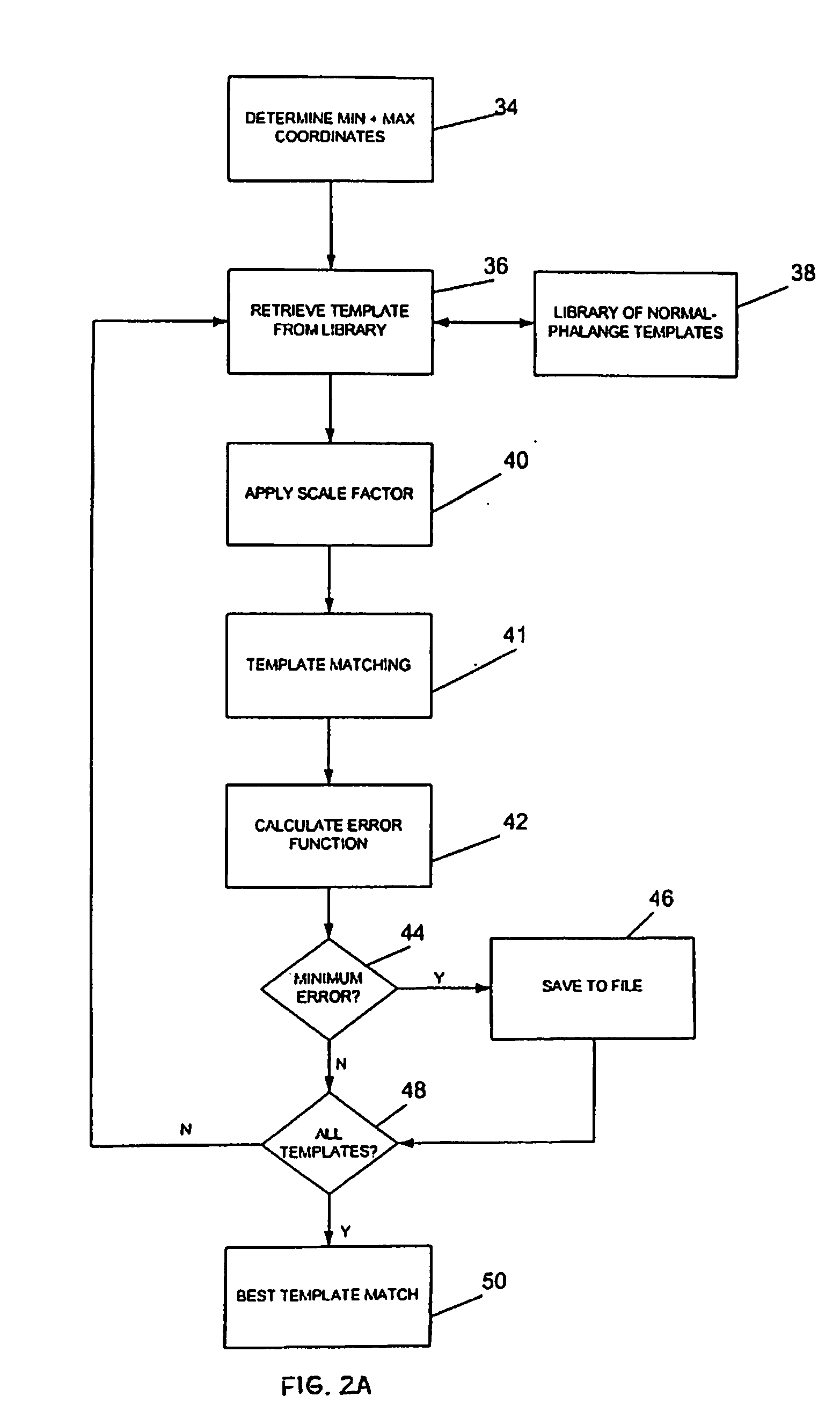
[0048]FIGS. 2A and 2B show a flow diagram of steps performed by the machine-readable storage medium embodying computer-readable code of the invention, in carrying out the method of Embodiment 1. As indicated by box 34 in the figure, the coordinates that are determined from the x-ray images are the coordinates at the left and right edges of the bone at its narrowest width (Min), and the apical coordinates corresponding to coordinates at the ends of a line through the widest portion of the phalange (Max) adjacent one or both joints of that phalange, for example, the greatest widths at the top and bottom joint regions of the middle flange.
[0049] The coordinates are determined by using the left and right bone edge contours to find the minimum bone width of the middle phalange. Starting from the first coordinate of the left bone edge contour, the program finds the corresponding coordinate that has the same Y coordinate from the right bone edge contour. The w...
embodiment 2
B. EMBODIMENT 2
Measuring Joint Bone Erosion
[0069] Erosion happens in early stages in RA patients and it rapidly gets worse in the first few years of the disease. When a patient is first seen, the physician's objective is to detect the existence and the severity of bone erosion. At this time the contours of the interested bones are obtained for the first time and saved as baseline results for future monitoring purpose. Embodiment 2 described in this section and Embodiment 3 described in the next sections are designed to assess the extent of bone loss in the joint region of a selected bone, e.g., phalange, using normal-bone contours to as reference contours. In follow-up visits, the patient's own earlier x-ray images may be used as the reference contours, for assessing the change in bone erosion over time. This approach is detailed below as Embodiment 4.
[0070] When the patient's bone contours are obtained for the first time, there are no pre-existing contours to reference in the dat...
embodiment 3
C. EMBODIMENT 3
Measuring Joint Bone Erosion
[0075] The method in this embodiment uses a portion of MP or PP templates to predict or estimate a normal contour (without erosion). Then, the current contour can be compared against the estimated contour to determine the existence and severity if the erosion.
[0076] From a MP or PP template, the algorithm defines two landmark points (described below in detailed steps) and applies a matching algorithm only on the contour points between them. By “copying over” the shape of the template's contour—the points between the two landmarks—for the area of interest (the area the erosion is supposed to occur), it is possible to partially overlay two contours and compare. The algorithm is shown in flow diagram in FIG. 7.
[0077] As a first step, indicated at box 92 in FIG. 7, the program finds the coordinates for an apical point P, as carried out in Embodiment 2, and the coordinates for the minimum width of the phalange (points N), as discussed below. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| joint space width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com