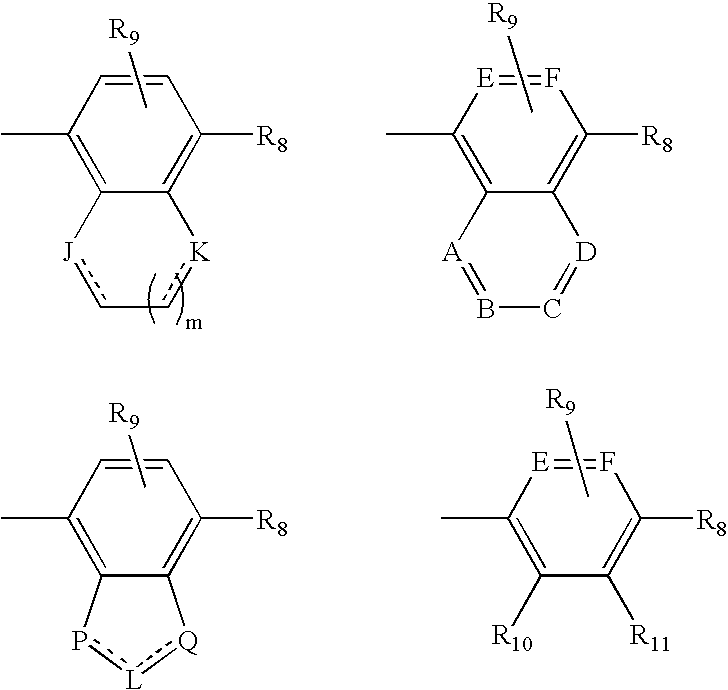
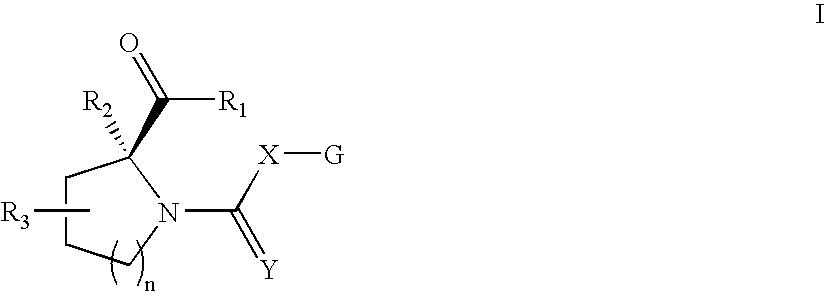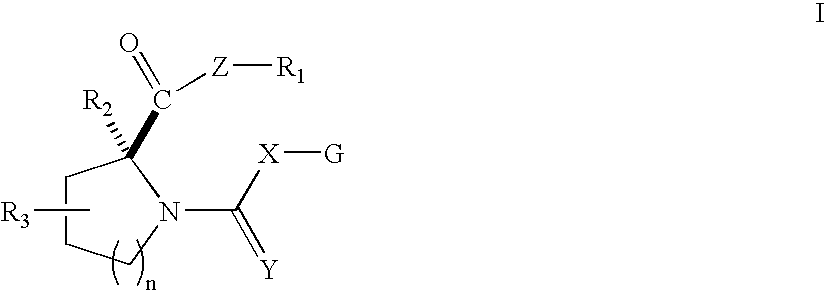Open-chain prolyl urea-related modulators of androgen receptor function
a prolyl urea and open-chain technology, applied in the field of open-chain prolyl urea and thiourea related compounds, can solve the problems of adverse effects on male sexual function, liver damage, prostate cancer, etc., to prevent the catabolic side effects of glucocorticoids, maintain muscle strength and function, and reduce bone density or growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1-(4-Cyano-2-ethyl-3-(trifluoromethyl)phenyl-1-carbamoyl)-3-hydroxy-pyrrolidine-2-carboxylic acid methyl ester
[0155]
Step 1. N-(4-Chloro-3-trifluoromethylphenyl)-2,2-dimethylpropionamide
[0156]
[0157] To a solution of 15.0 g (76.7 mmol) of 4-chloro-3-(trifluoromethyl)aniline in 200 mL of anhydrous THF cooled to 0-5° C. was added 11.7 mL (84.4 mmol) of NEt3 followed by 10.4 mL (84.4 mmol) of pivaloyl chloride over 30 min. The ice bath was removed, and the mixture was stirred for 1 h, then diluted with ether and filtered. The filtrate was washed with water (2×) and brine, dried (MgSO4), filtered and concentrated. The residue was triturated with hexanes and the solid filtered and dried to give 20.4 g (95%) of the Step 1 compound. 1H NMR (DMSO-d6) δ 1.22 (s, 9H), 7.63 (d, J=8.8, 1H), 8.00 (dd, J=2.8, 8.8, 1H), 8.24 (d, J=2.2, 1H), 9.62 (s, 1H); 13C NMR (DMSO-d6)δ26.94, 118.6, 121.7, 123.6, 124.6, 126.4, 126.6, 131.7, 138.9, 177.1; HPLC-1: 3.62 min retention time, column: Phenominex ODS C...
example 2
1-(4-Cyanonaphthalen-1-ylcarbamoyl)-3-hydroxy-pyrrolidine-2-carboxylic acid methyl ester
[0166]
Step 1. 4-Isocyanato-naphthalene-1-carbonitrile
[0167]
[0168] The title compound was prepared from 4-cyanonaphthylamine in a similar fashion to that described in Step 5 of Example 1 for the preparation of an aryl isocyanate from an aryl amine.
Step 2. 1-(4-Cyanonaphthalen-1-ylcarbamoyl)-3-hydroxypyrrolidine-2-carboxylic acid methyl ester
[0169] To a suspension of the Step 1 compound (0.51 g, 1.96 mmol) in CH2Cl2 (2 mL) cooled to 0° C. was added i-Pr2EtN (0.37 mL, 2.16 mmol). After stirring at 0° C. for 20 min, cis-3-hydroxyproline methyl ester (0.34 g, 1.75 mmol) in CH2Cl2 (1 mL) solution was added, along with 4 Å molecular sieves (0.5 g) and the resulting mixture stirred at rt until urea formation was complete (˜1 h). The reaction mixture was then loaded on a silica gel column, eluted with 50% EtOAc / hexane, and 5% MeOH in EtOAc / hexane (1:1) to afford 436 mg of the title compound as a white...
example 3
1-(5-Chloro-6-cyano-pyridin-3-ylcarbamoyl)-3-hydroxypyrrolidine-2-carboxylic acid methyl ester
[0170]
Step 1. 5-Amino-3-chloropyridine-2-carbonitrile
[0171]
[0172] To a solution of 3-chloro-5-nitro-pyridine-2-carbonitrile (0.1 g, 0.549 mmol) in 50% EtOAc and 40% H2O and 10% HOAc was added iron powder (0.1 g). The reaction mixture was stirred at 60° C. for 5 min until the amine was formed. The mixture was washed with H2O and the EtOAc was removed under reduced pressure. The crude product was purified by chromatography, eluted with 30% EtOAc in hexane and 2% MeOH in EtOAc / hexane to afford the title compound (0.66 g) as a white solid.
Step 2. 3-Chloro-5-isocyanatopyridine-2-carbonitrile
[0173]
[0174] To a stirring suspension of the Step 1 compound (0.066 g, 0.43 mmol) and Et3N (0.09 mL, 0.86 mmol) in THF (4 mL) cooled to 0° C. was added a solution of phosgene (20%) in toluene (0.085 mL, 0.86 mmol). After addition, the mixture was stirred at rt for 10 min. The mixture was then concentrated...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com