Electrochemical Method for the Production of Betulin Aldehyde
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
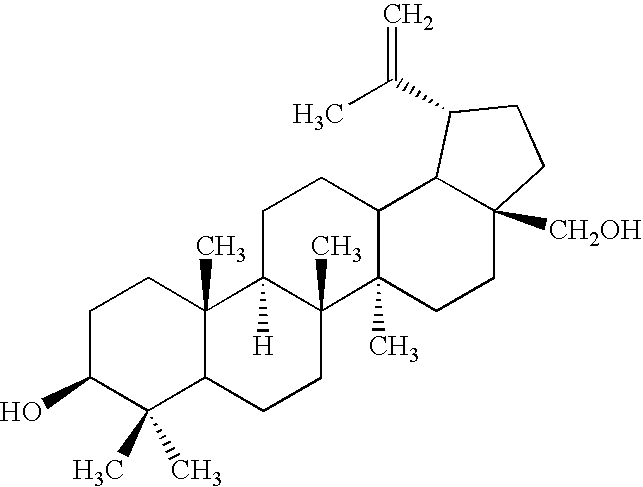
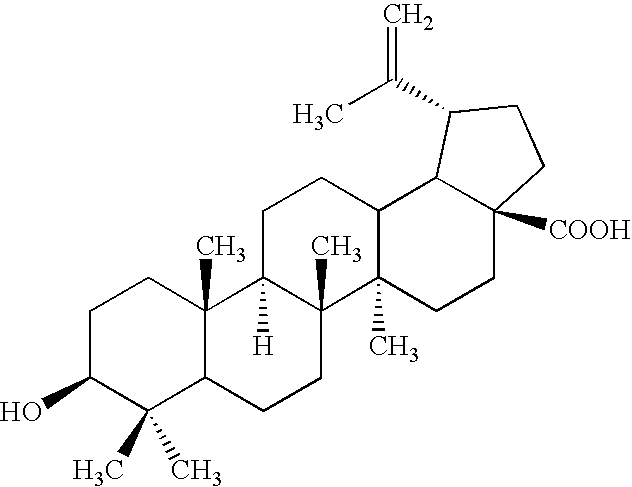
Preparation of Betulin Aldehyde from Betulin
[0139]450 ml of N,N-dimethylformamide, 20 g of betulin and 4 g of tetra-n-butylammonium perchlorate were loaded into a 500 ml Duran reaction vessel equipped with a heating mantle, mechanical stirrer, reflux condenser, glass-jacketed thermocouple and electrode bank of 3 flat platinum gauze anodes (50×50 mm) and 2 copper plate cathodes of the same size, clamped through 3 mm Teflon spacers. The mixture was heated up to 65° C. with stirring, then 20 ml of water and finally 1 g of TEMPO were added to mixture. After this, sufficient voltage was applied to the electrodes to maintain a 350-400 mA current. The process started at 2.9 V, then the voltage increased to 3.4-3.8 V and remained in this range for about 10 hours. At the end of the process the mixture turned from orange to colorless, and the voltage increased rapidly to 4 V and higher. The mixture was removed from the reactor to a flask, evaporated to about 200 ml, then diluted with 400 ml o...
example 2
Preparation of Betulin Aldehyde from Betulin
[0140]2500 ml of N,N-dimethylformamide, 120 g of betulin and 20 g of tetraethylammonium p-toluenesulfonate were loaded into a 2500 ml Duran reaction vessel equipped with a heating mantle, mechanical stirrer, reflux condenser, glass-jacketed thermocouple and an electrode bank of a cylindrical platinum gauze anode of total surface area 550 cm2 and 2 coaxial copper gauze cathodes, clamped through 4 mm Teflon rings. The mixture was heated up to 70° C. with stirring, then 100 ml of water and finally 5 g of TEMPO were added to the mixture. After this, sufficient voltage was applied on the electrodes to get a current of 3 A. The process started at 2.9 V, then the voltage increased to 3.4-3.8 V and remained in this range for about 25 hours. At the end of the process the mixture turned from orange to colorless, and the voltage increased rapidly to 4 V and higher. The mixture was removed from the reactor to a flask and diluted with 4 L of water with...
example 3
Preparation of Betulin Aldehyde from Betulin
[0141]2500 ml of N,N-dimethylacetamide, 120 g of betulin and 20 g of tetraethylammonium p-toluenesulfonate were loaded into the reaction vessel of Example 2. The mixture was heated up to 60° C. with stirring, then 60 ml of water and finally 5 g of TEMPO were added to mixture. After this, sufficient voltage was applied to the electrodes to get a current of 1.7 A. The voltage started at 2.6 V, then increased to 3.1±0.1 V and maintained in this range for about 35 hours. After the voltage further increased to 3.5 Volts, the process was maintained 8 hours more at constant voltage. At the end of process the mixture turned from orange to colorless. The mixture was removed from the reactor to a flask and diluted with 4 L of water with stirring. The white deposit was filtered after the mixture cooled down, washed with water, and dried at 70° C. Yield: 120 g of a mixture of 92% betulin aldehyde and 8% betulin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com