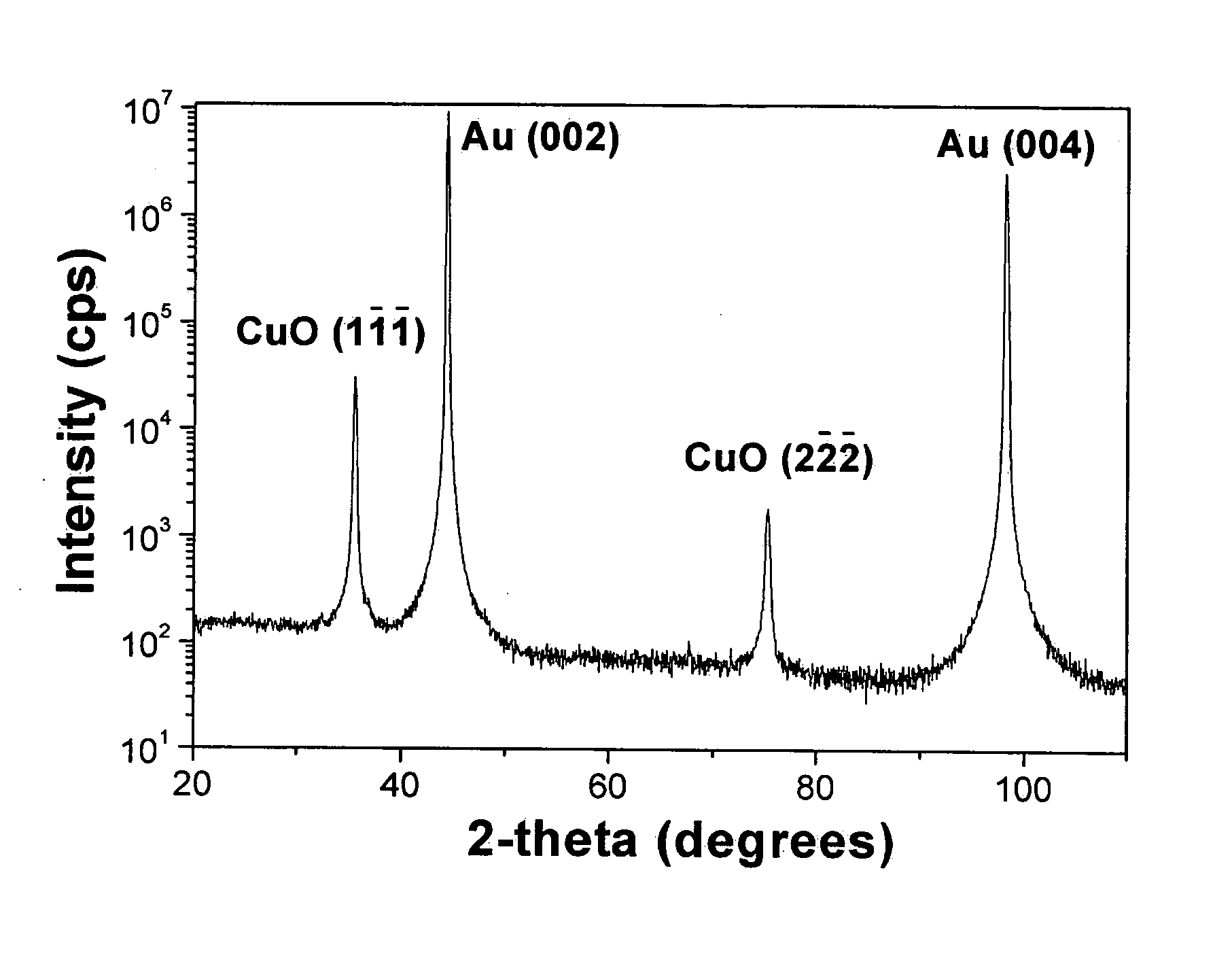
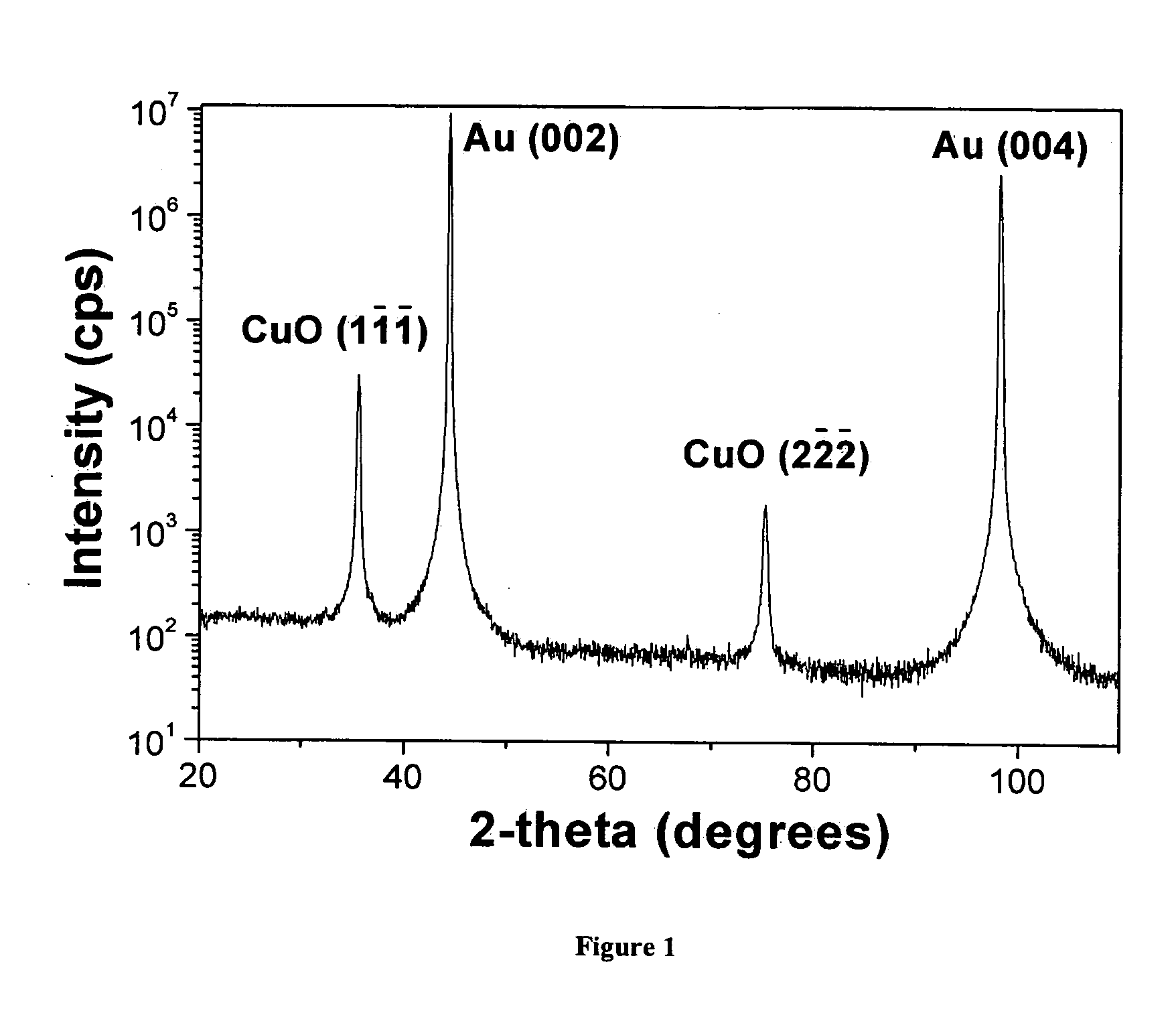
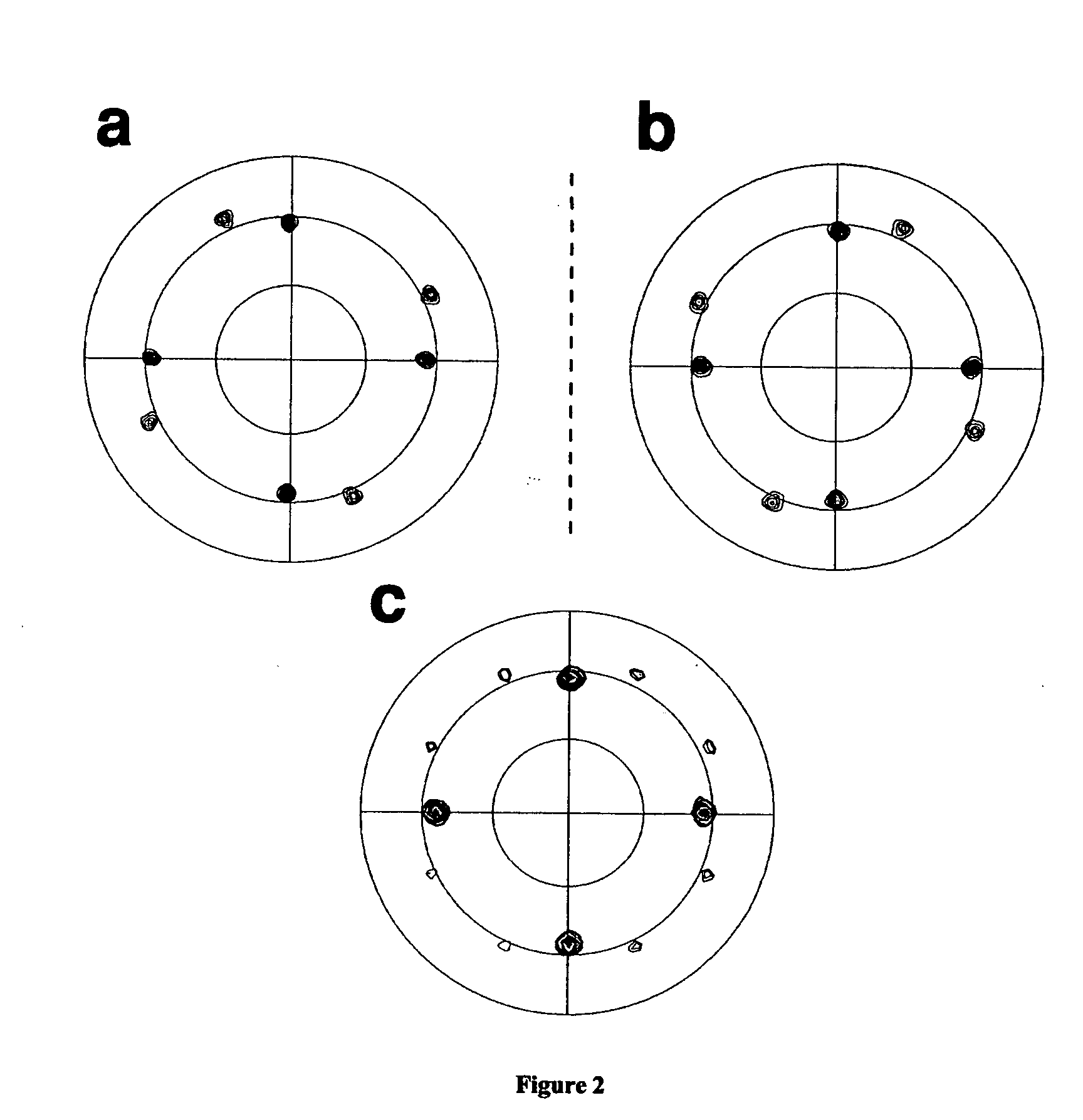
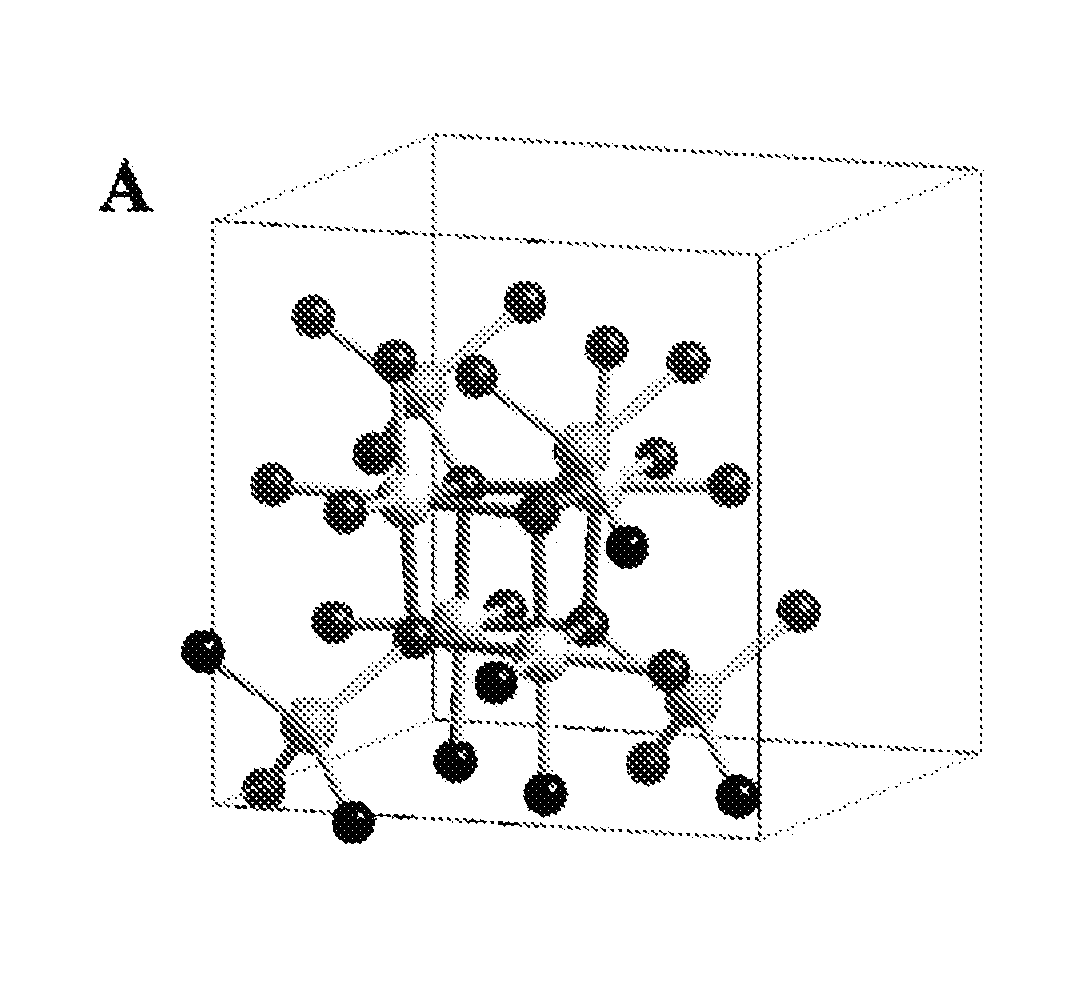
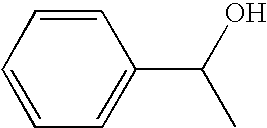
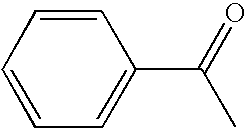
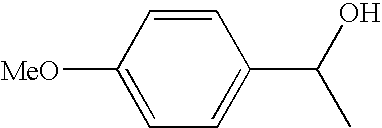
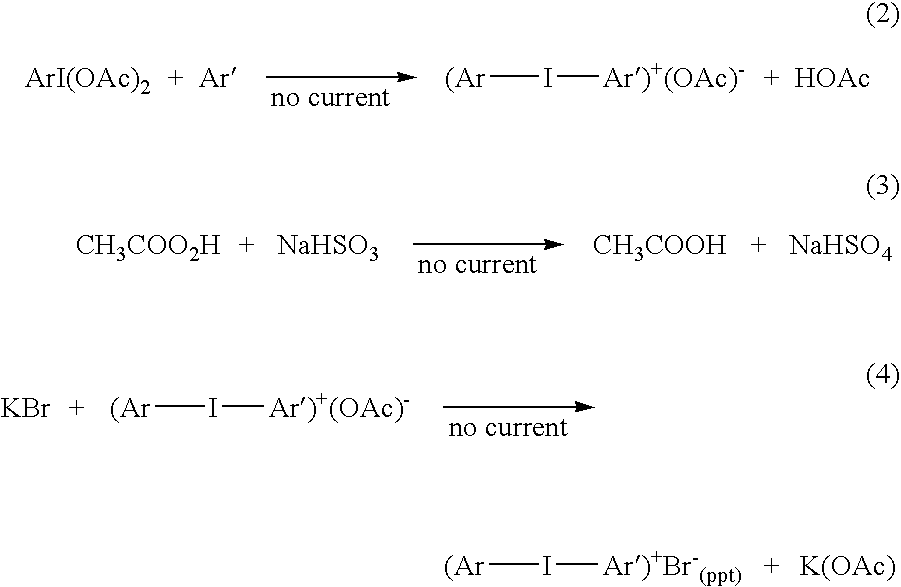Patents
Literature
69results about "Electrolytic organic oxidation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
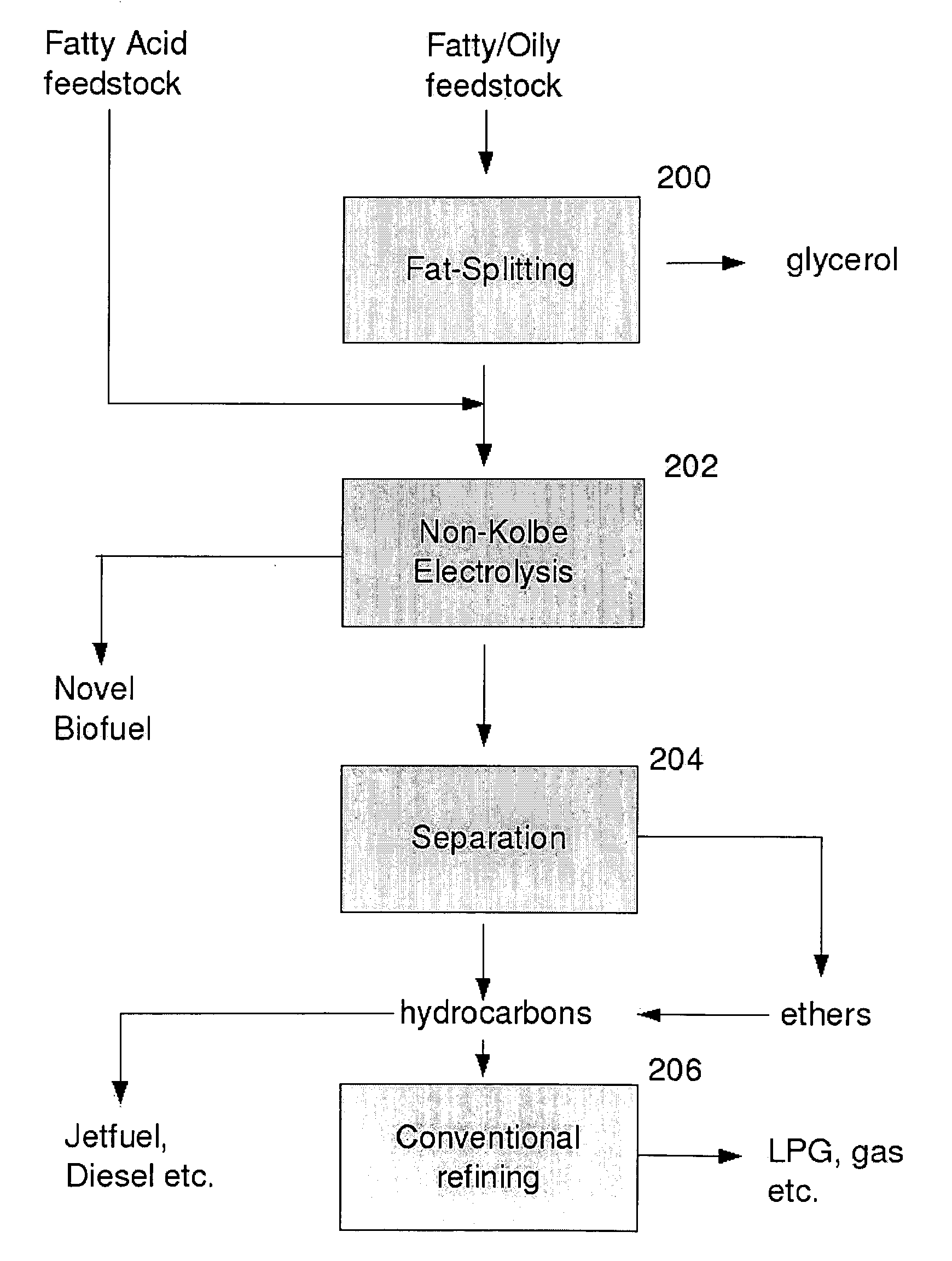
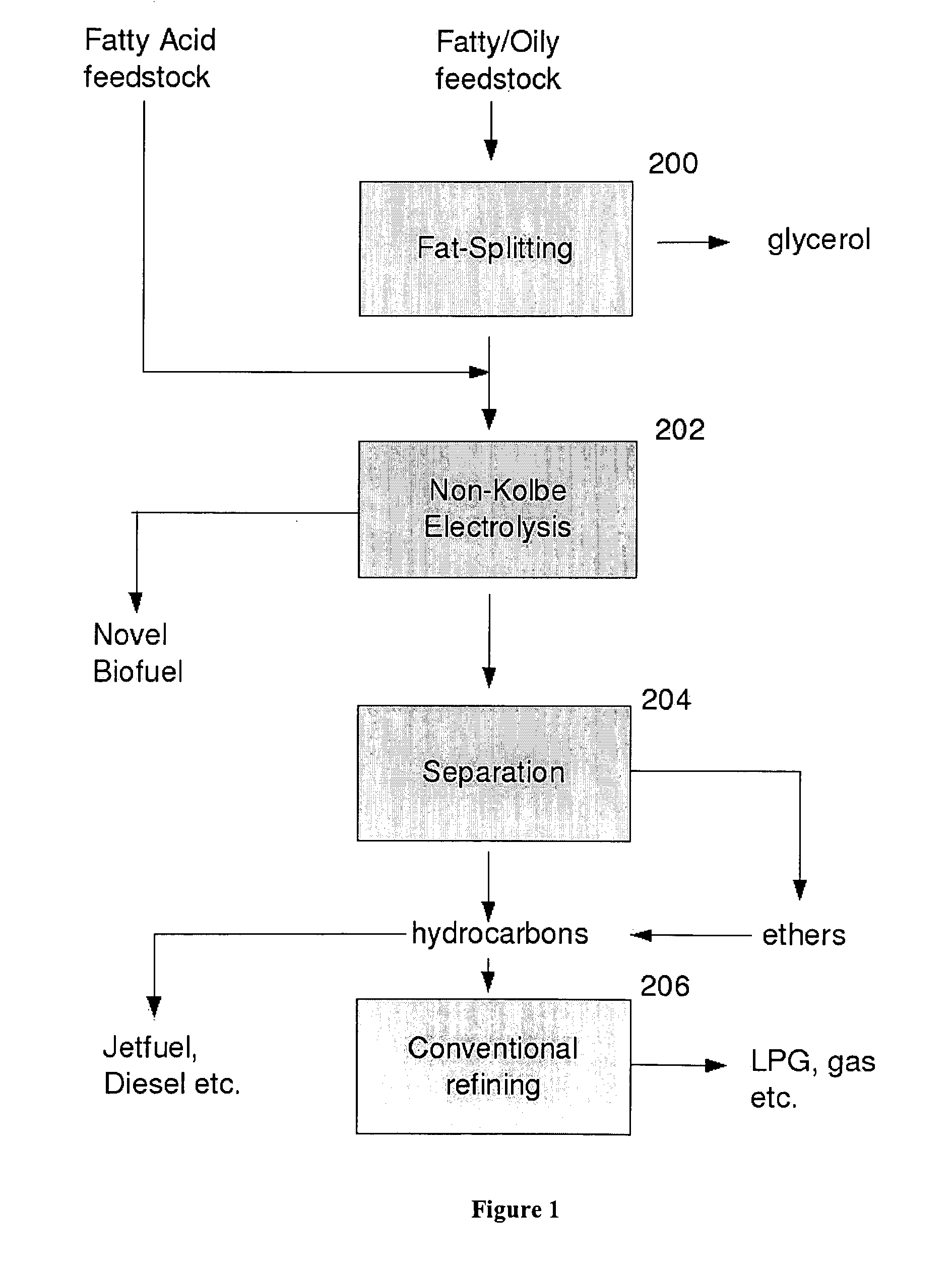
Production of fuel from chemicals derived from biomass
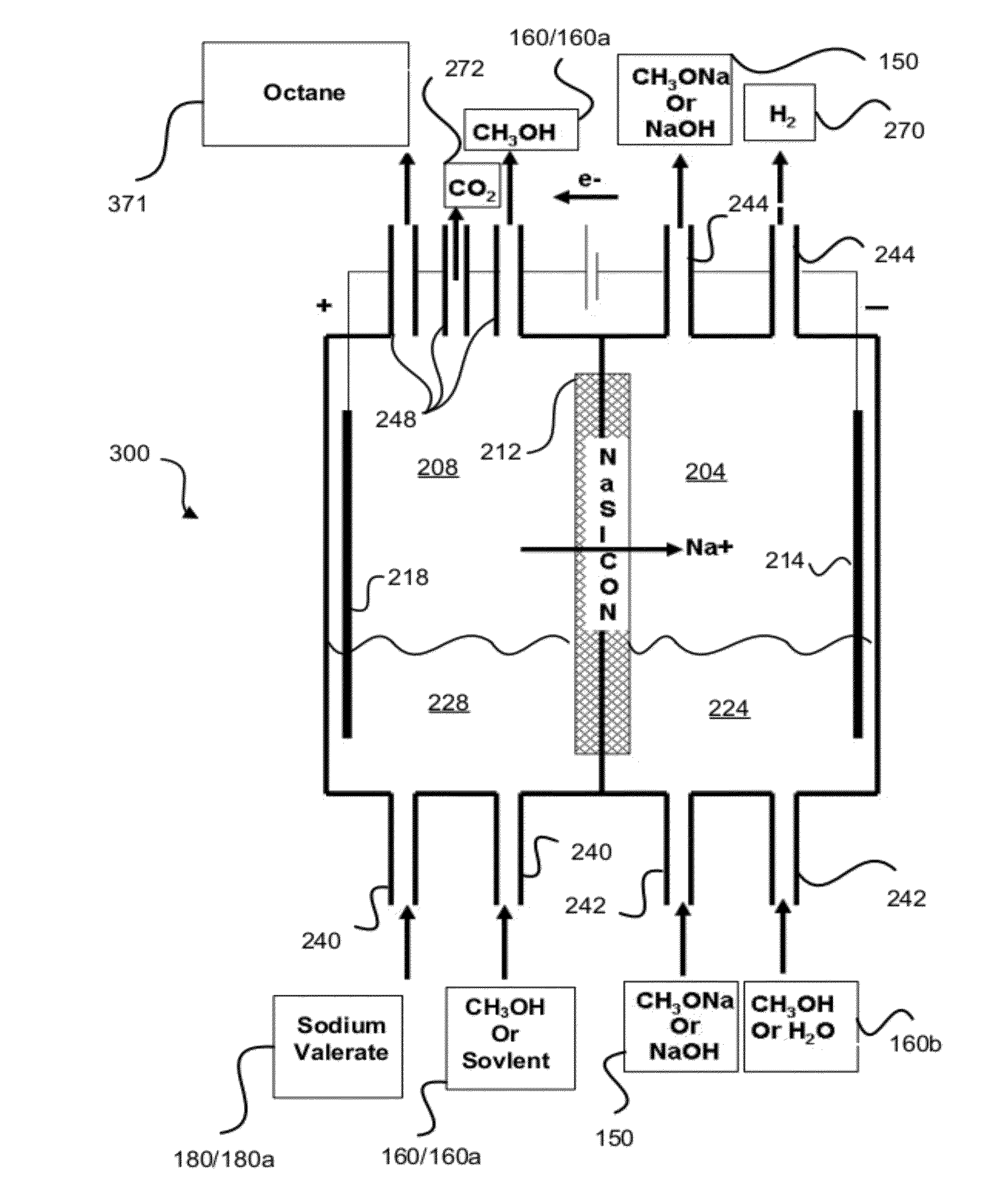
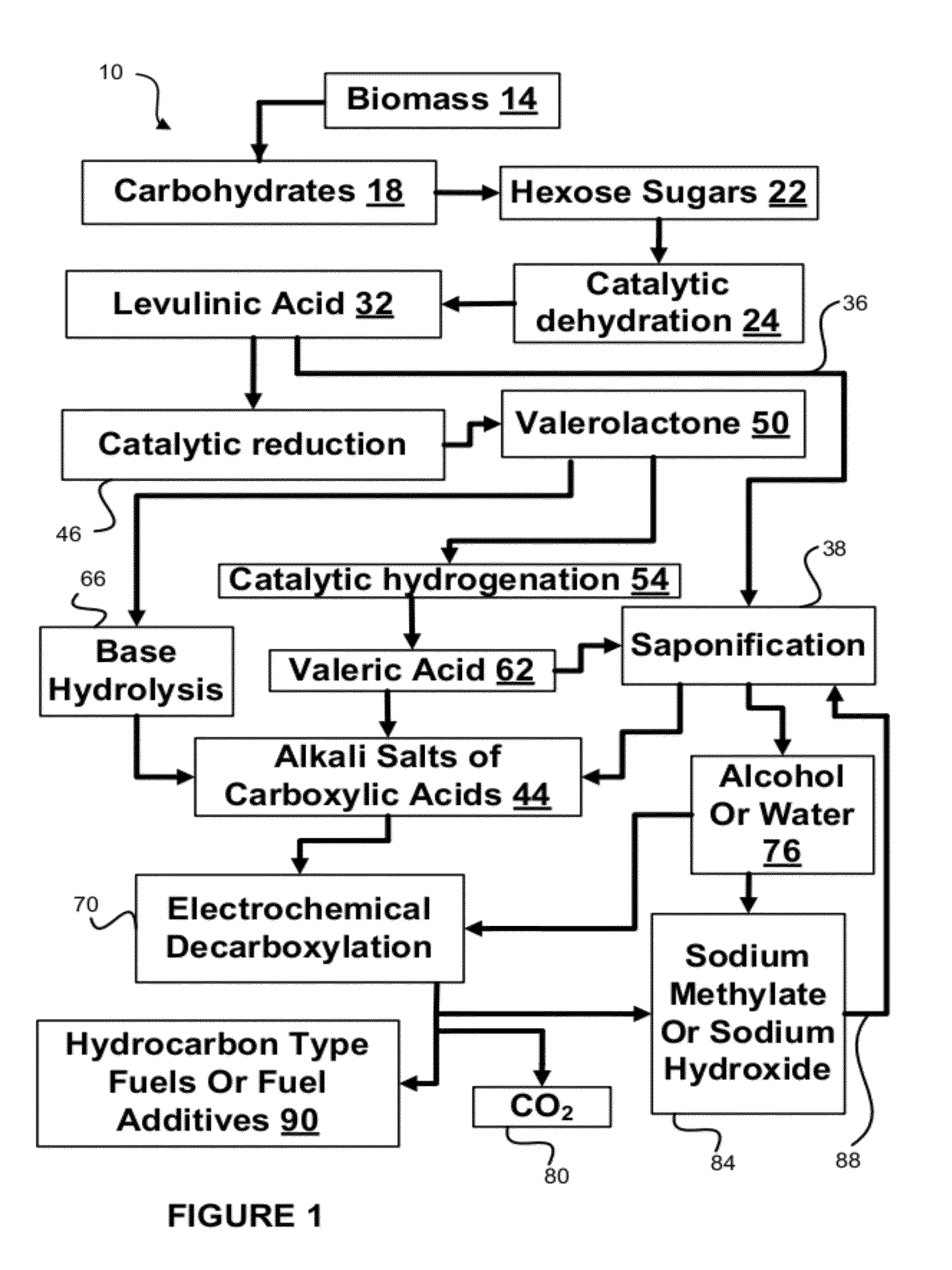
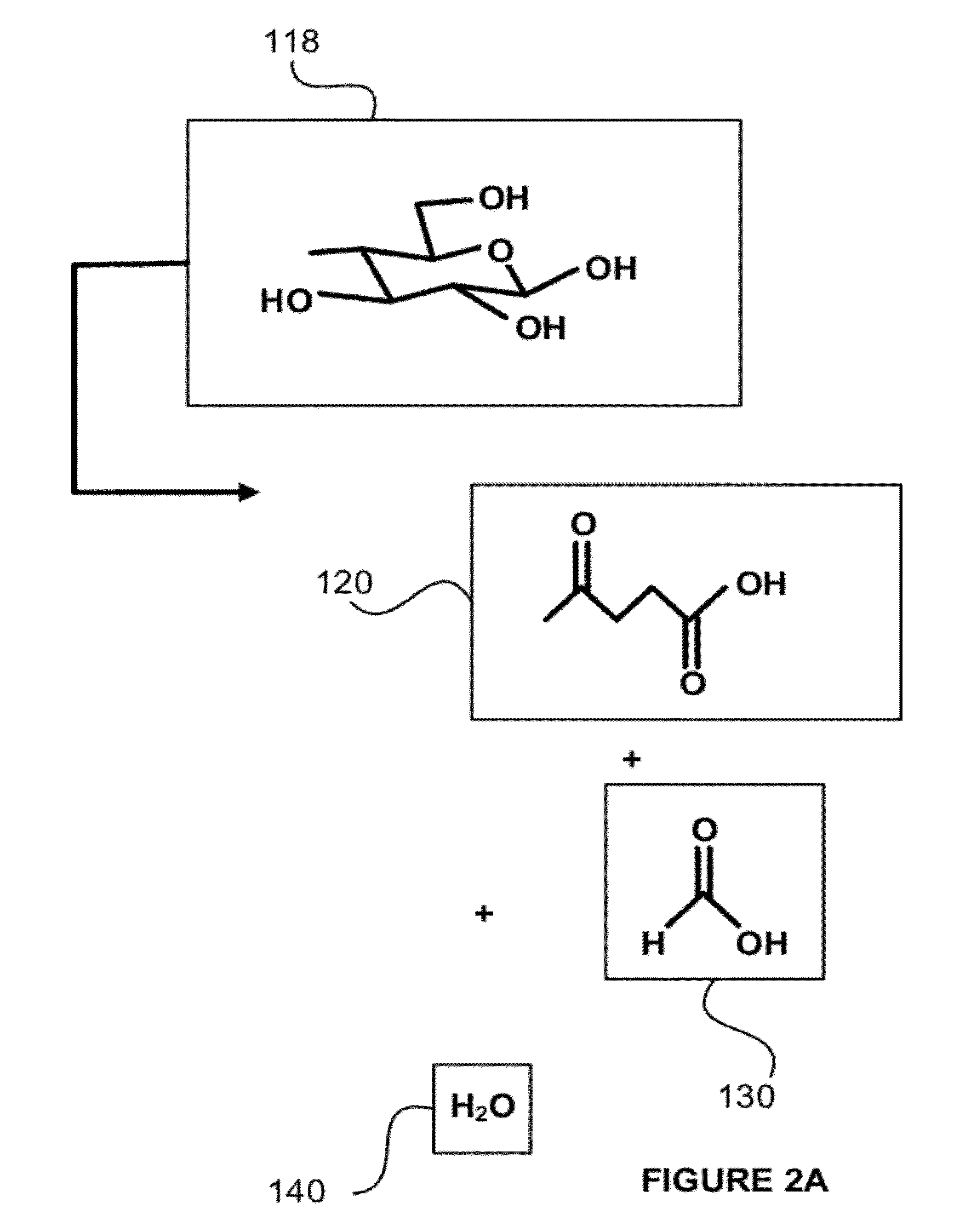
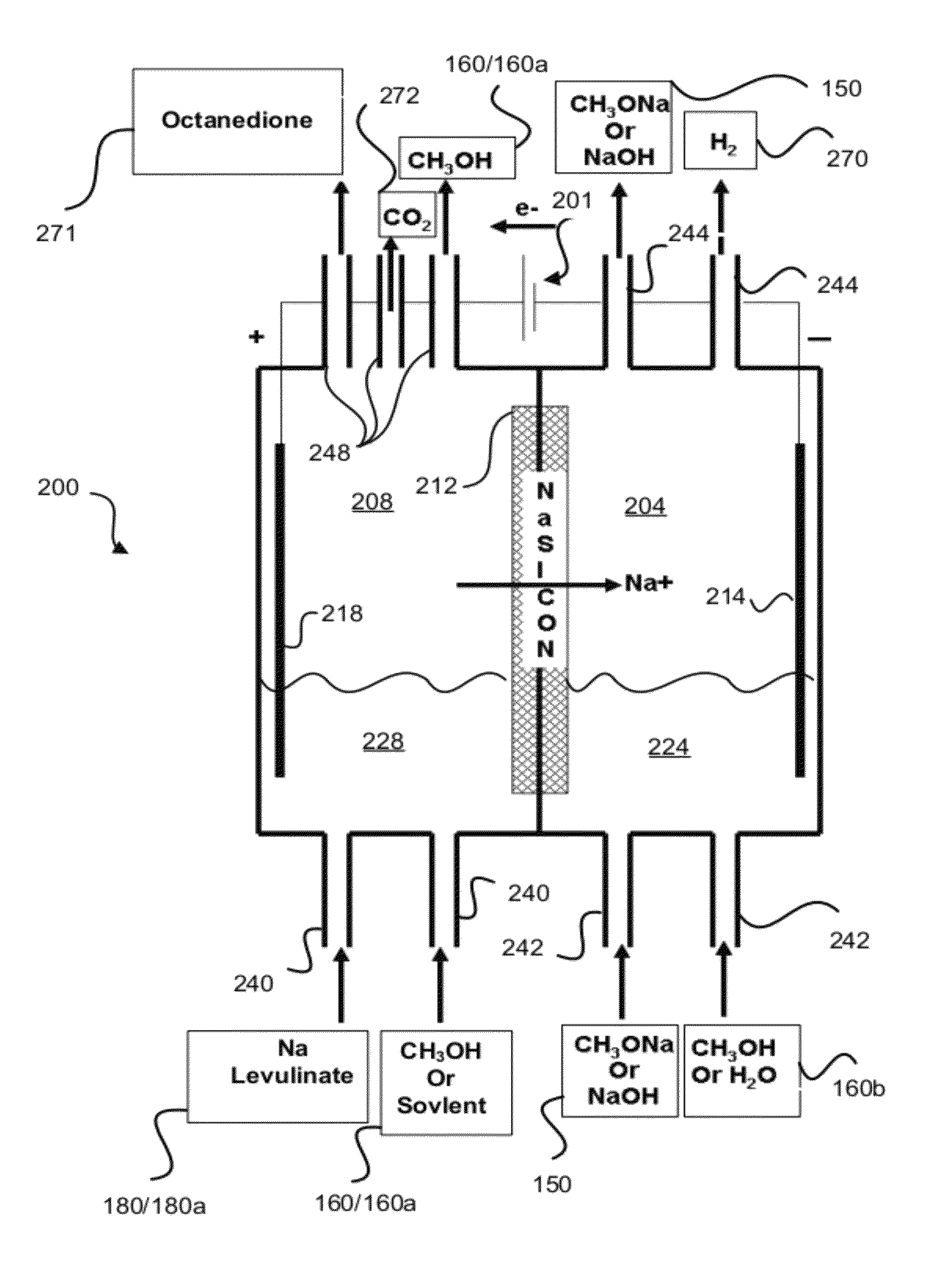
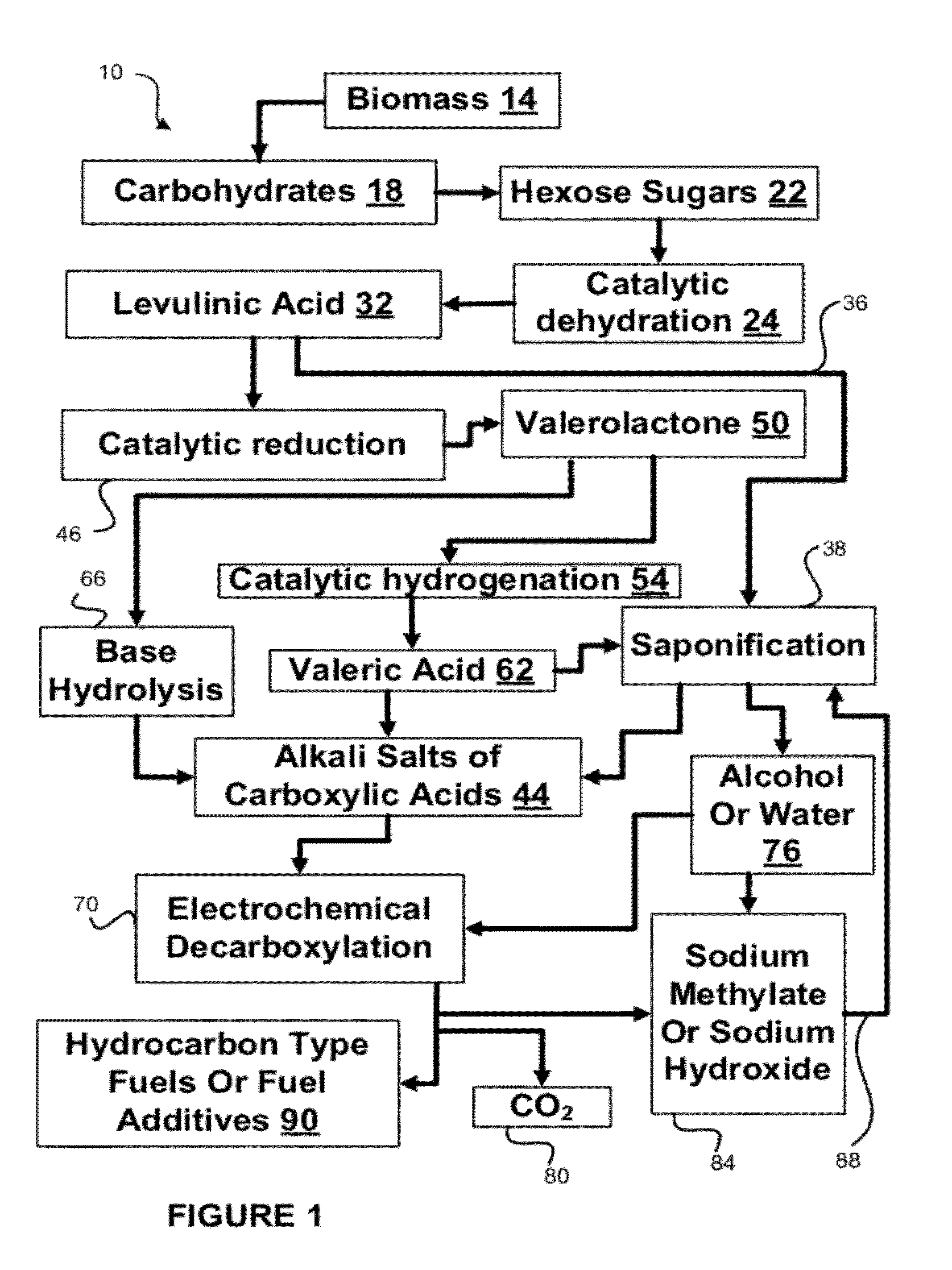
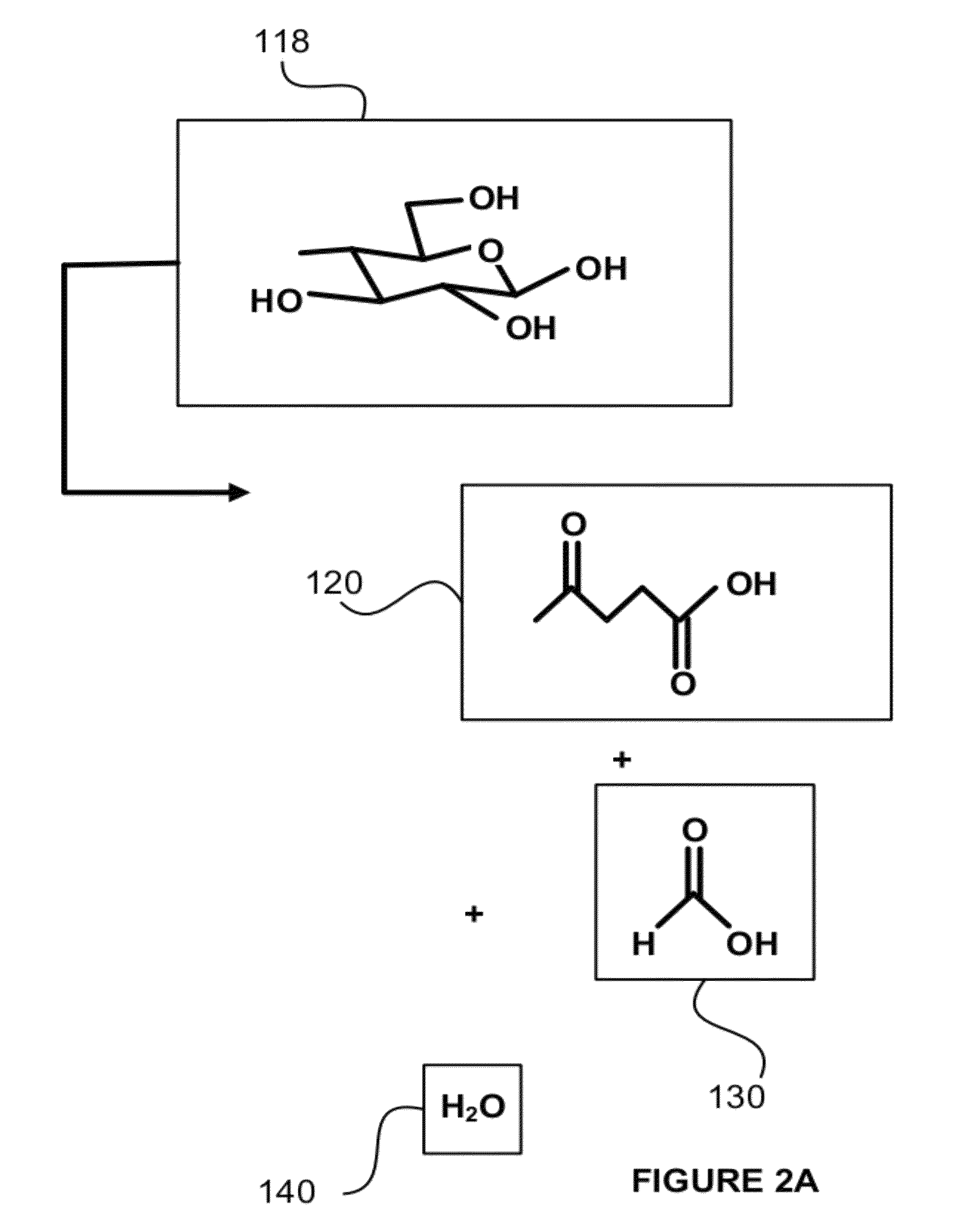
Hydrocarbons may be formed from six carbon sugars. This process involves obtaining a quantity of a hexose sugar. The hexose sugar may be derived from biomass. The hexose sugar is reacted to form an alkali metal levulinate, an alkali metal valerate, an alkali metal 5-hydroxy pentanoate, or an alkali metal 5-alkoxy pentanoate. An anolyte is then prepared for use in a electrolytic cell. The anolyte contains the alkali metal levulinate, the alkali metal valerate, the alkali metal 5-hydroxy pentanoate, or the alkali metal 5-alkoxy pentanoate. The anolyte is then decarboxylated. This decarboxylating operates to decarboxylate the alkali metal levulinate, the alkali metal valerate, the alkali metal 5-hydroxy pentanoate, or the alkali metal 5-alkoxy pentanoate to form radicals, wherein the radicals react to form a hydrocarbon fuel compound.
Owner:ENLIGHTEN INNOVATIONS INC
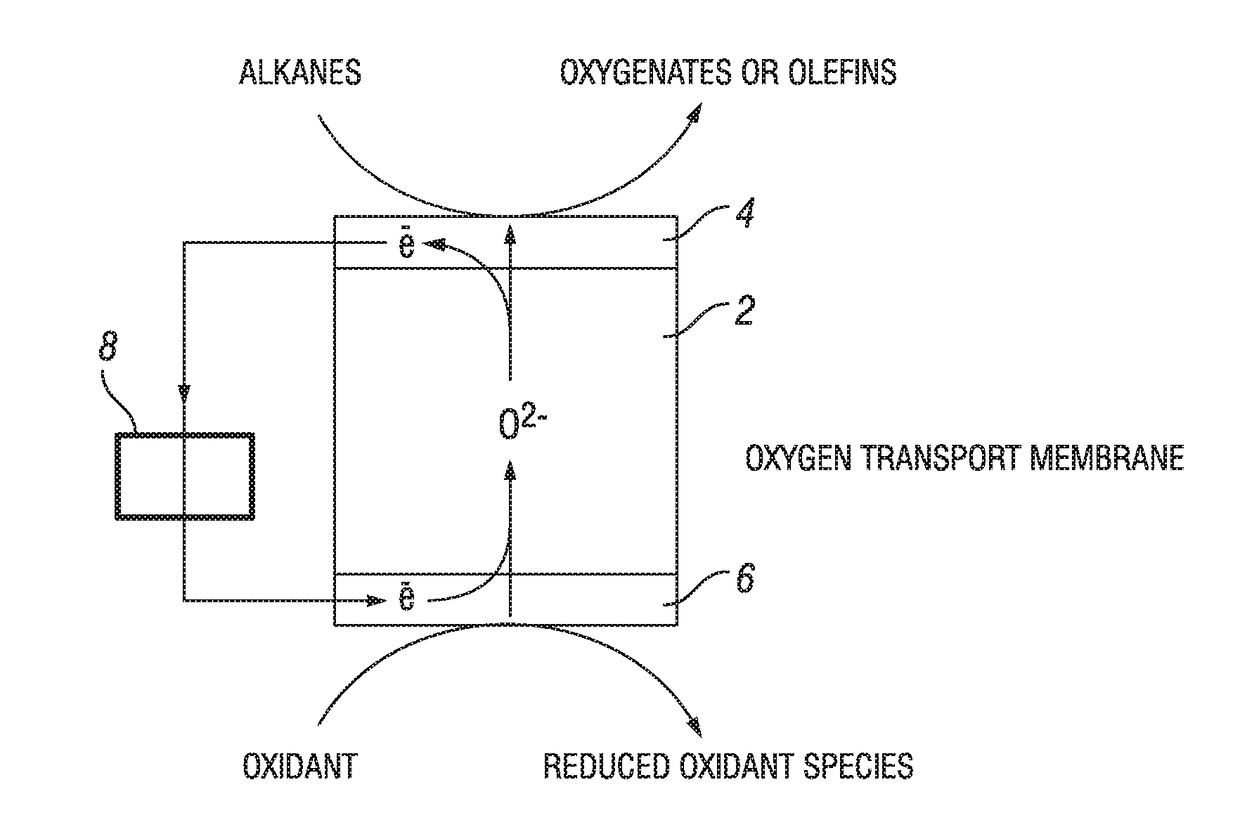
Reactor, process, and system for the oxidation of gaseous streams
InactiveUS20170247803A1Semi-permeable membranesElectrolysis componentsElectrical conductorLiquid fuel
A reactor and process capable of concurrently producing electric power and selectively oxidizing gaseous components in a feed stream, such as hydrocarbons to unsaturated products, which are useful intermediates in the production of liquid fuels. The reactor includes an oxidation membrane, a reduction membrane, an electron barrier, and a conductor. The oxidation membrane and reduction membrane include an MIEC oxide. The electron barrier, located between the oxidation membrane and the reduction membrane, is configured to allow transmission of oxygen anions from the reduction membrane to the oxidation membrane and resist transmission of electrons from the oxidation membrane to the reduction membrane. The conductor conducts electrons from the oxidation membrane to the reduction membrane.
Owner:ECOCATALYTIC INC
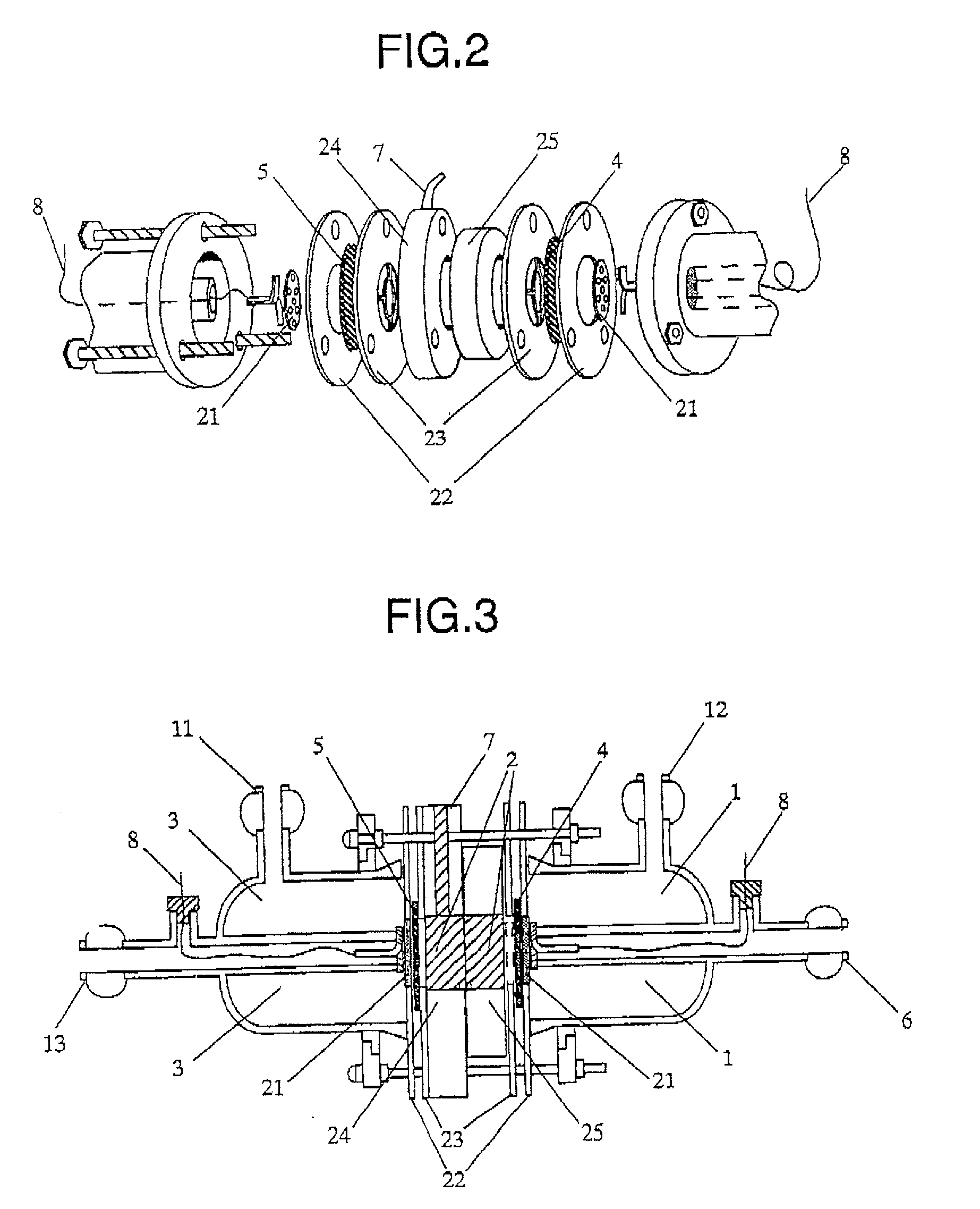
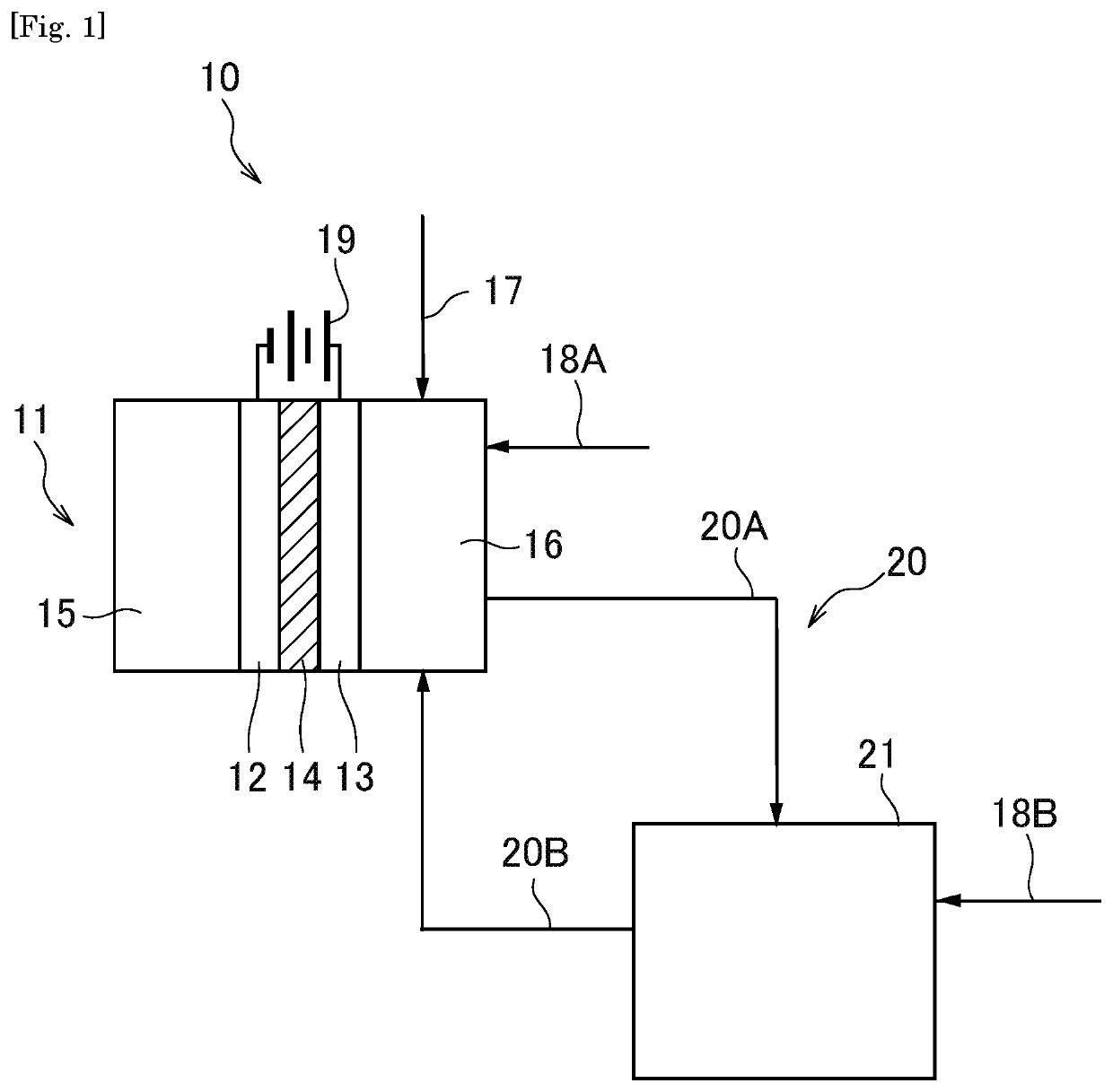
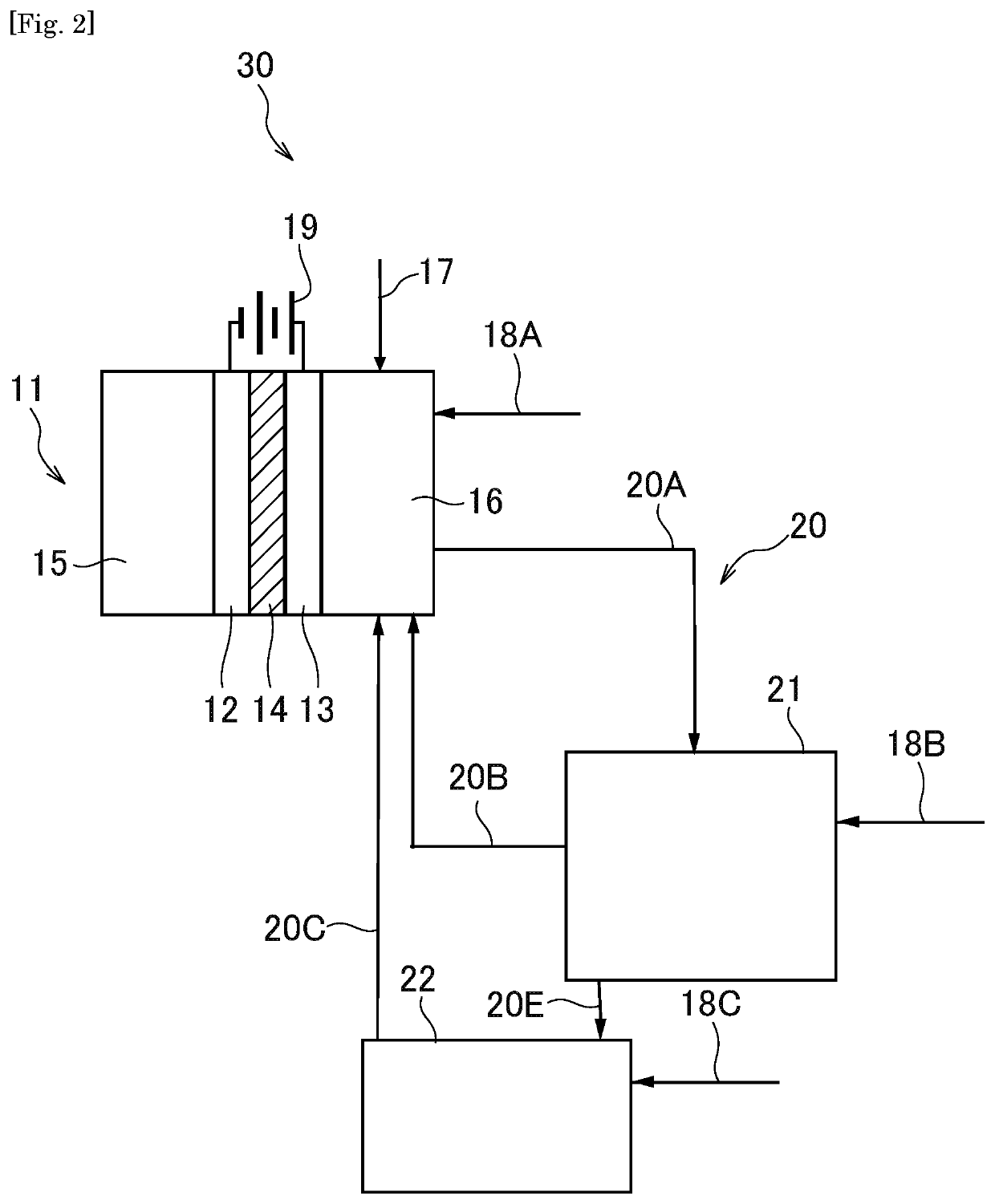
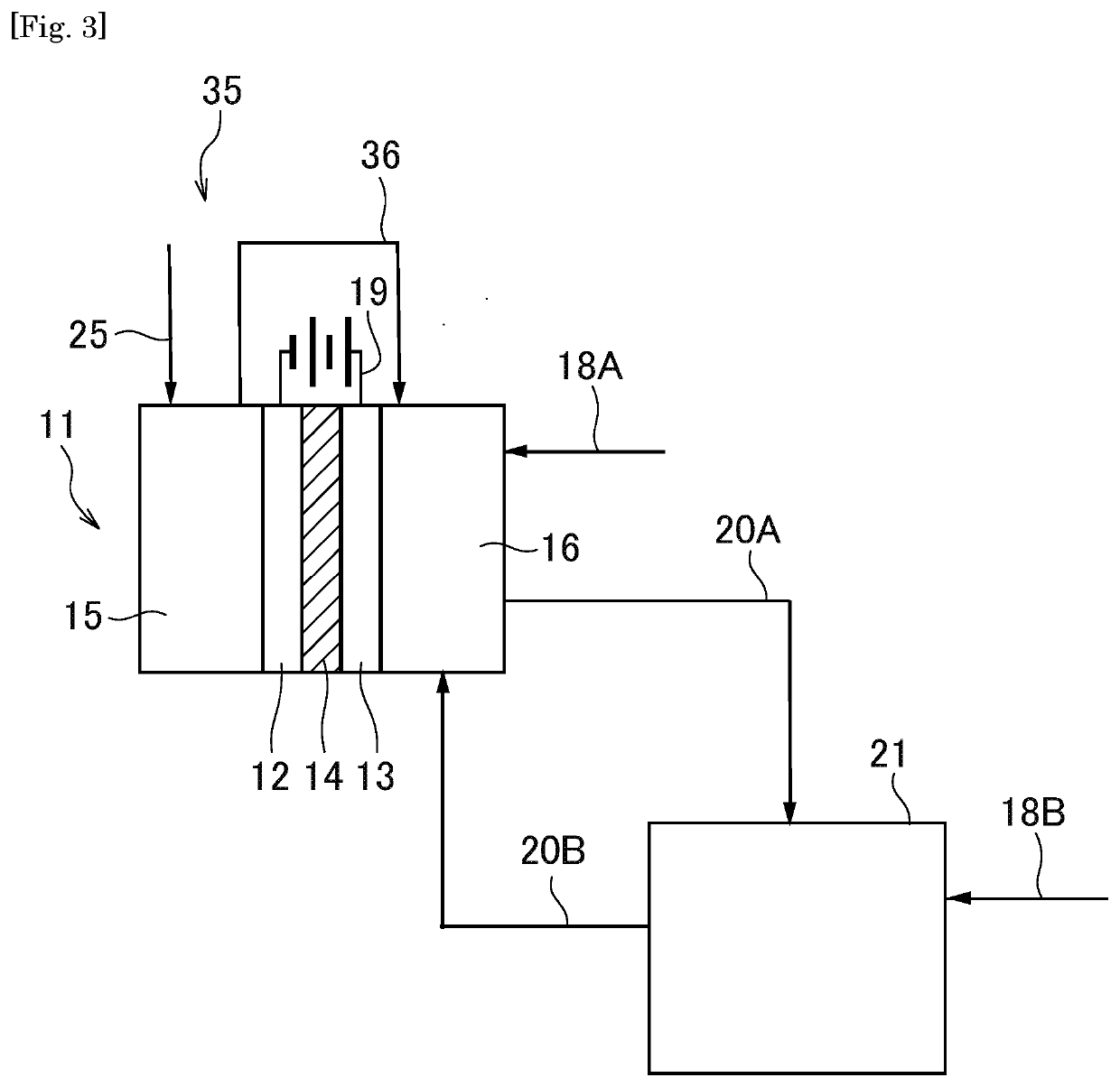
Organic electrolysis reactor for performing an electrolytic oxidation reaction and method for producing a chemical compound by using the same
Disclosed is an organic electrolysis reactor for performing an electrolytic oxidation reaction of a system comprising a substrate and a reductant, comprising: a casing; an anode which comprises an anode active material and which is ion-conductive or active species-conductive; a cathode which comprises a cathode active material and which is ion-conductive or active species-conductive; and means for applying a voltage between the anode and the cathode, wherein the means for applying a voltage is disposed in the outside of the casing and connected to the anode and the cathode, wherein the anode and the cathode are disposed in spaced relationship in the casing to partition the inside of the casing into an intermediate compartment between the anode and the cathode, and an anode compartment on the outside of the anode. Also disclosed is a method for producing a chemical compound by performing an electrolytic oxidation reaction of a system comprising a substrate and a reductant, using the organic electrolysis reactor mentioned above.
Owner:ASAHI KASEI KK
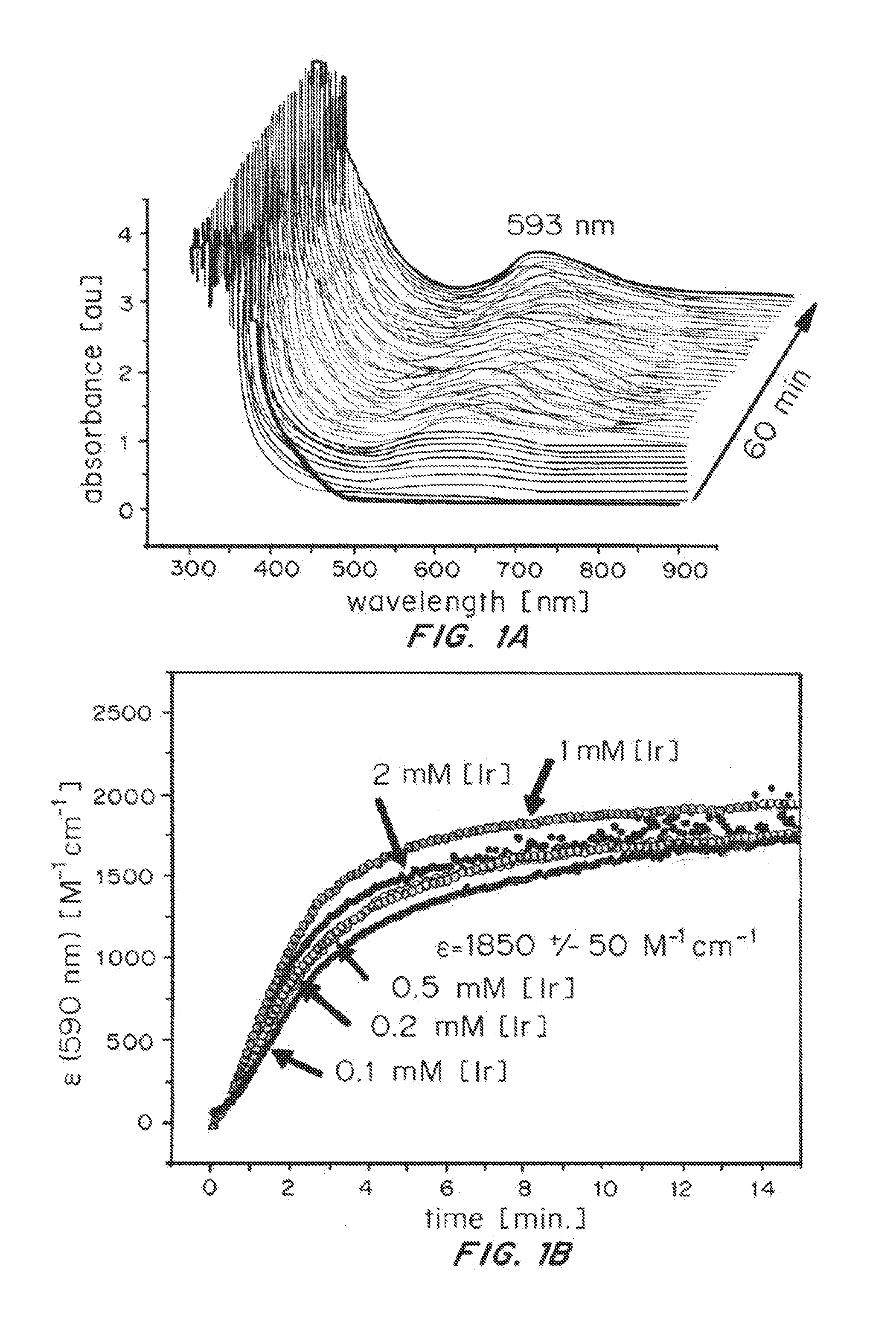
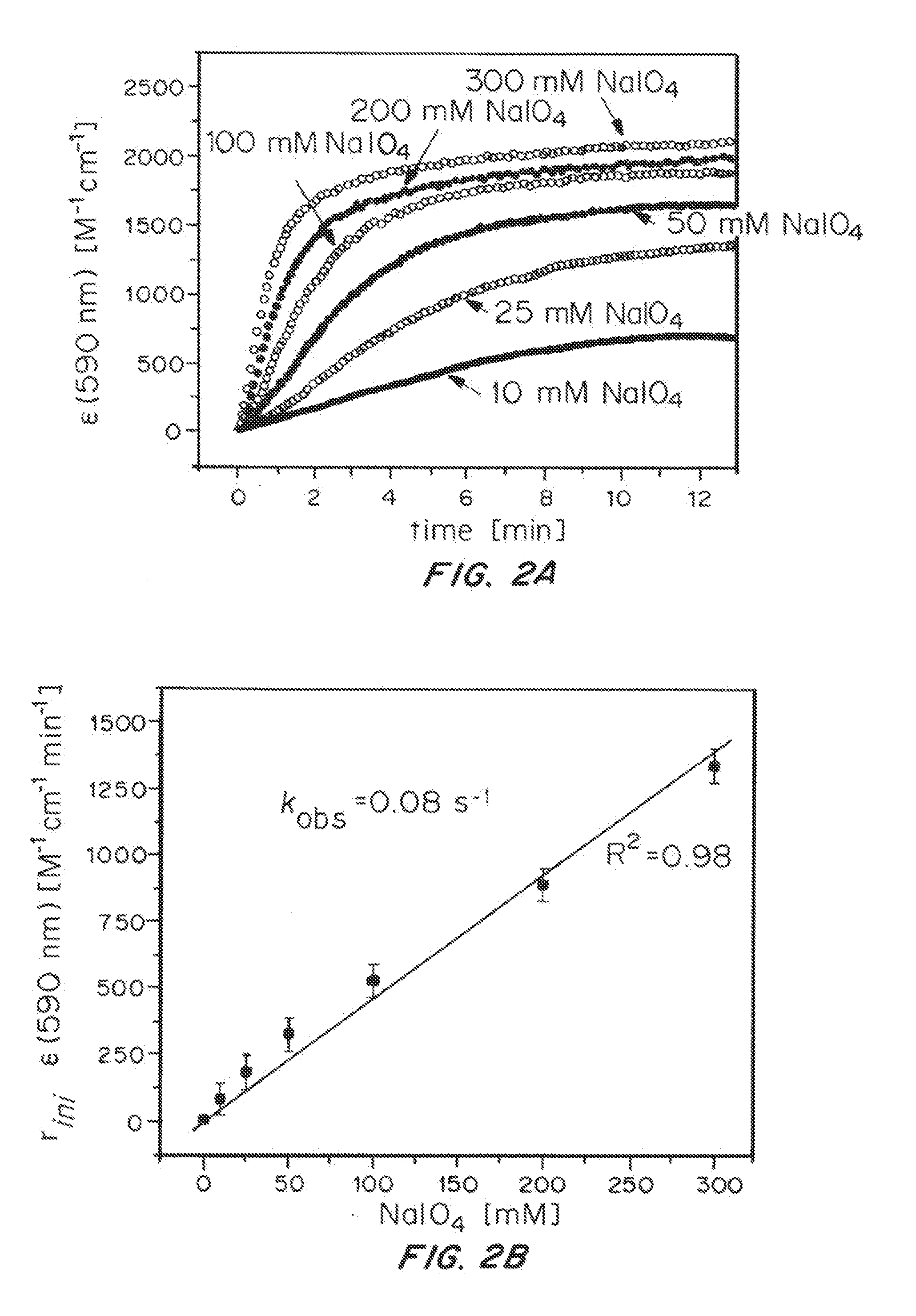
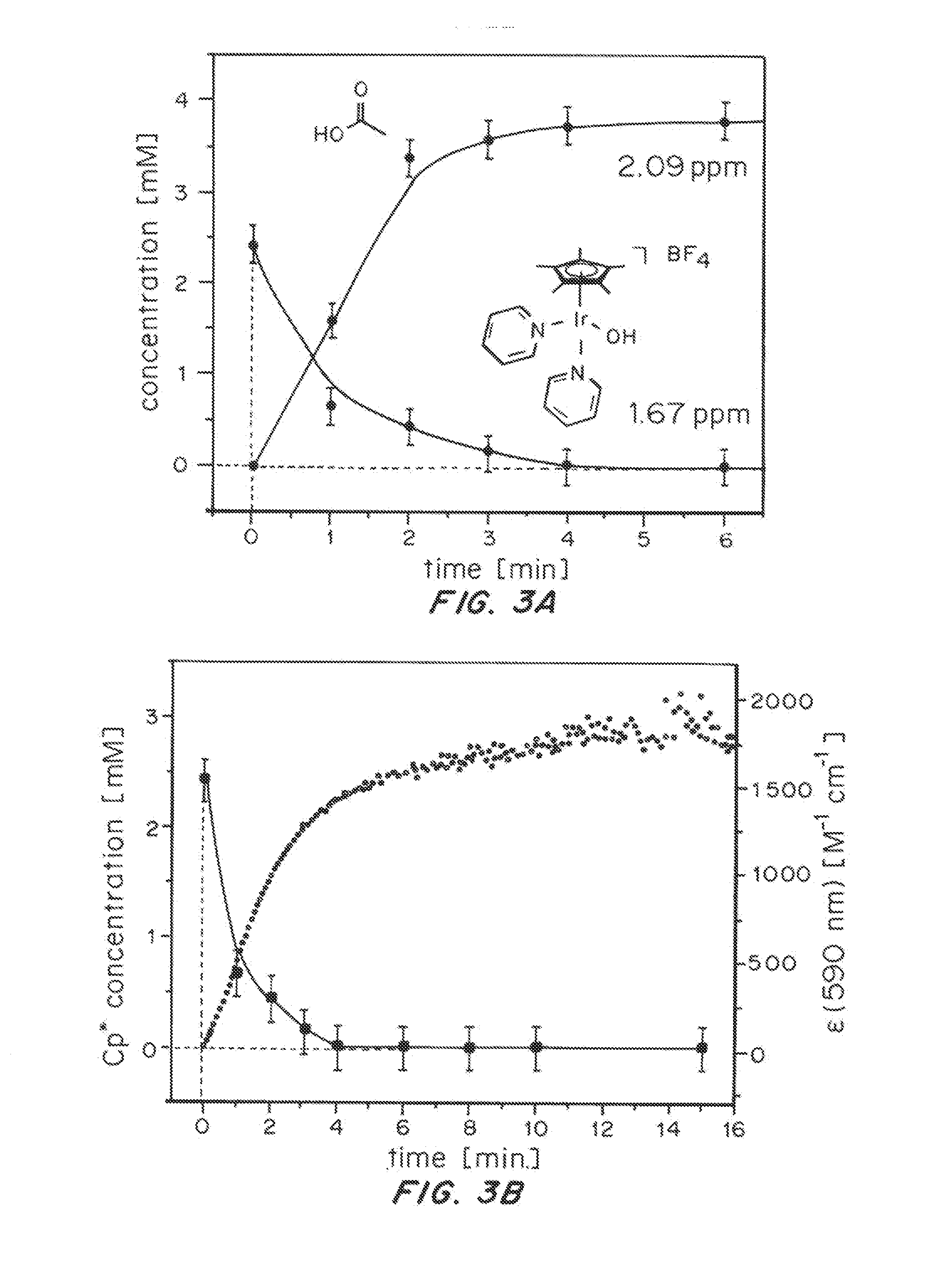
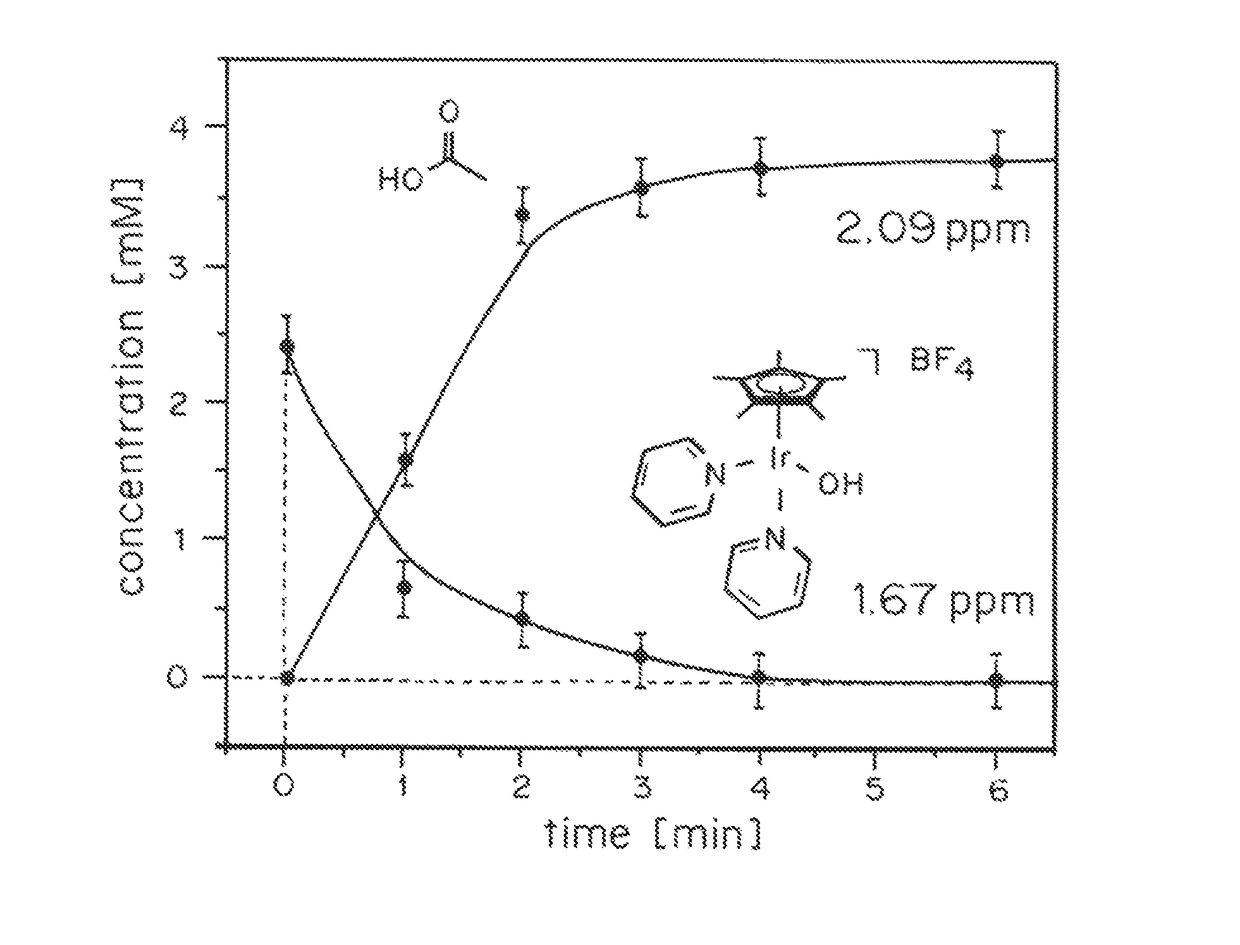
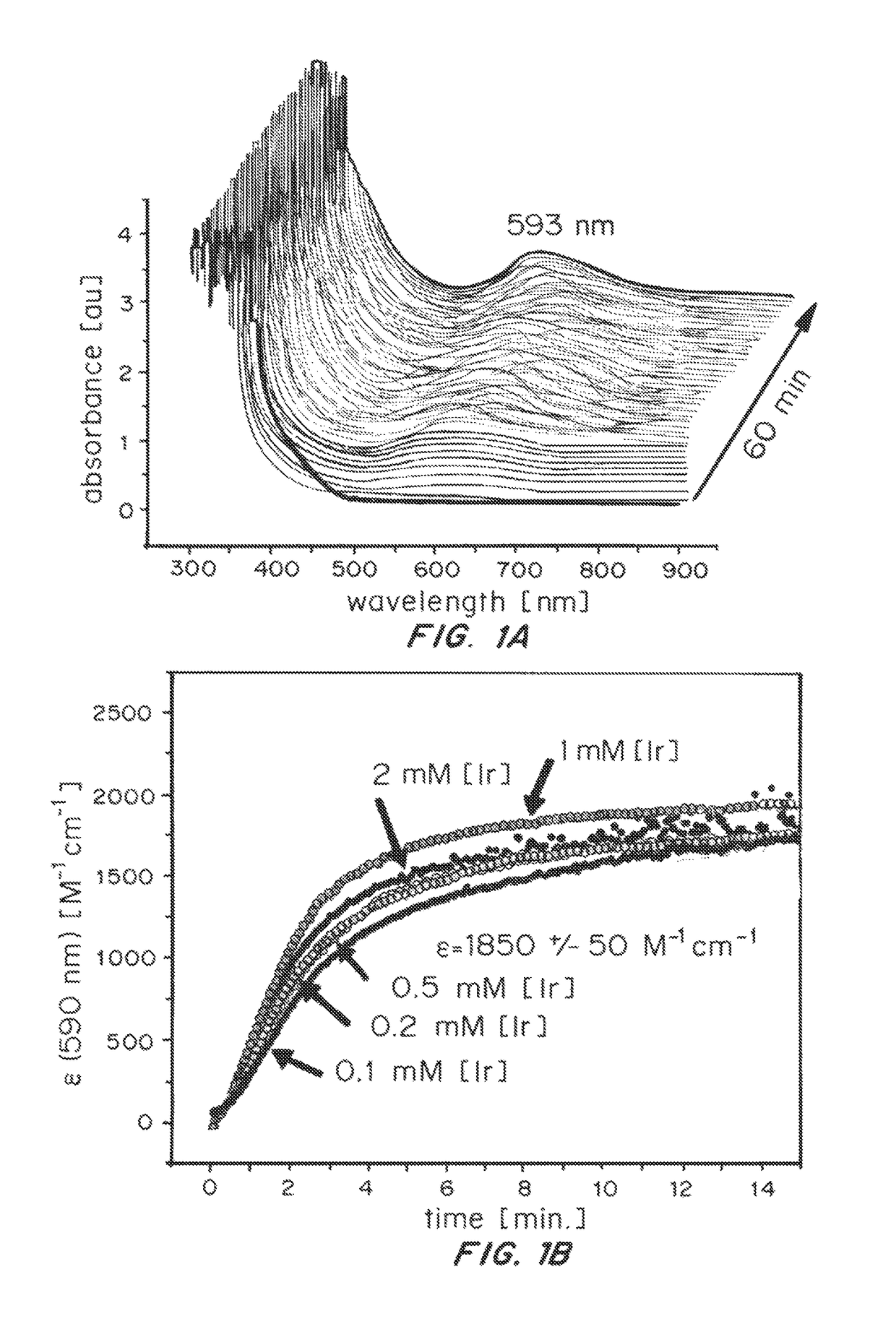
Iridium complexes for electrocatalysis
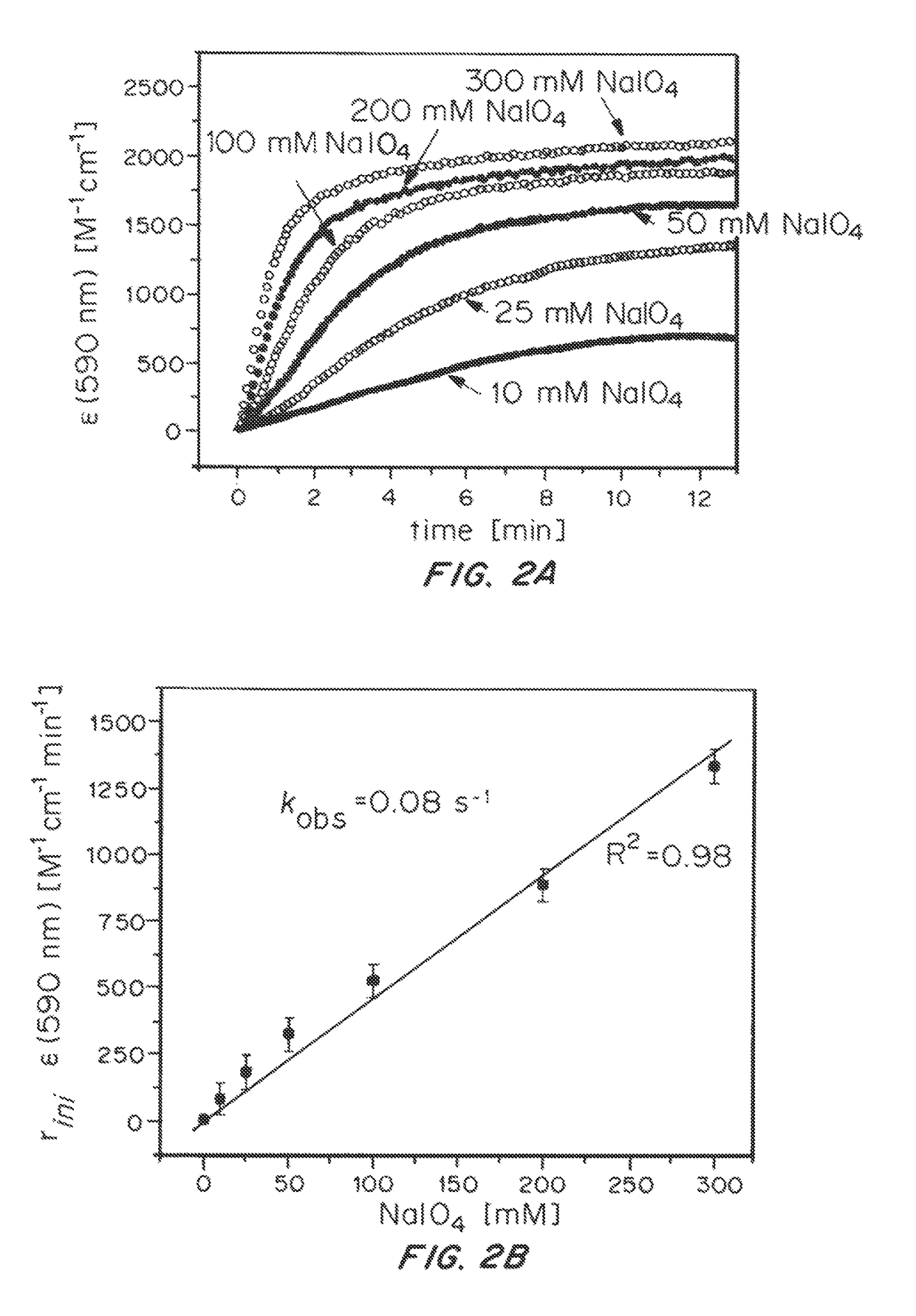
ActiveUS20150021194A1Improve stabilityConvenient bindingPlatinum group organic compoundsFrom normal temperature solutionsIridiumPentamethylcyclopentadiene
Solution-phase (e.g., homogeneous) or surface-immobilized (e.g., heterogeneous) electrode-driven oxidation catalysts based on iridium coordination compounds which self-assemble upon chemical or electrochemical oxidation of suitable precursors and methods of making and using thereof are. Iridium species such as {[Ir(LX)x(H2O)y(μ-O)]zm+}n wherein x, y, m are integers from 0-4, z and n from 1-4 and LX is an oxidation-resistant chelate ligand or ligands, such as such as 2(2-pyridyl)-2-propanolate, form upon oxidation of various molecular iridium complexes, for instance [Cp*Ir(LX)OH] or [(cod)Ir(LX)] (Cp*=pentamethylcyclopentadienyl, cod=cis-cis,1,5-cyclooctadiene) when exposed to oxidative conditions, such as sodium periodate (NaIO4) in aqueous solution at ambient conditions.
Owner:YALE UNIV
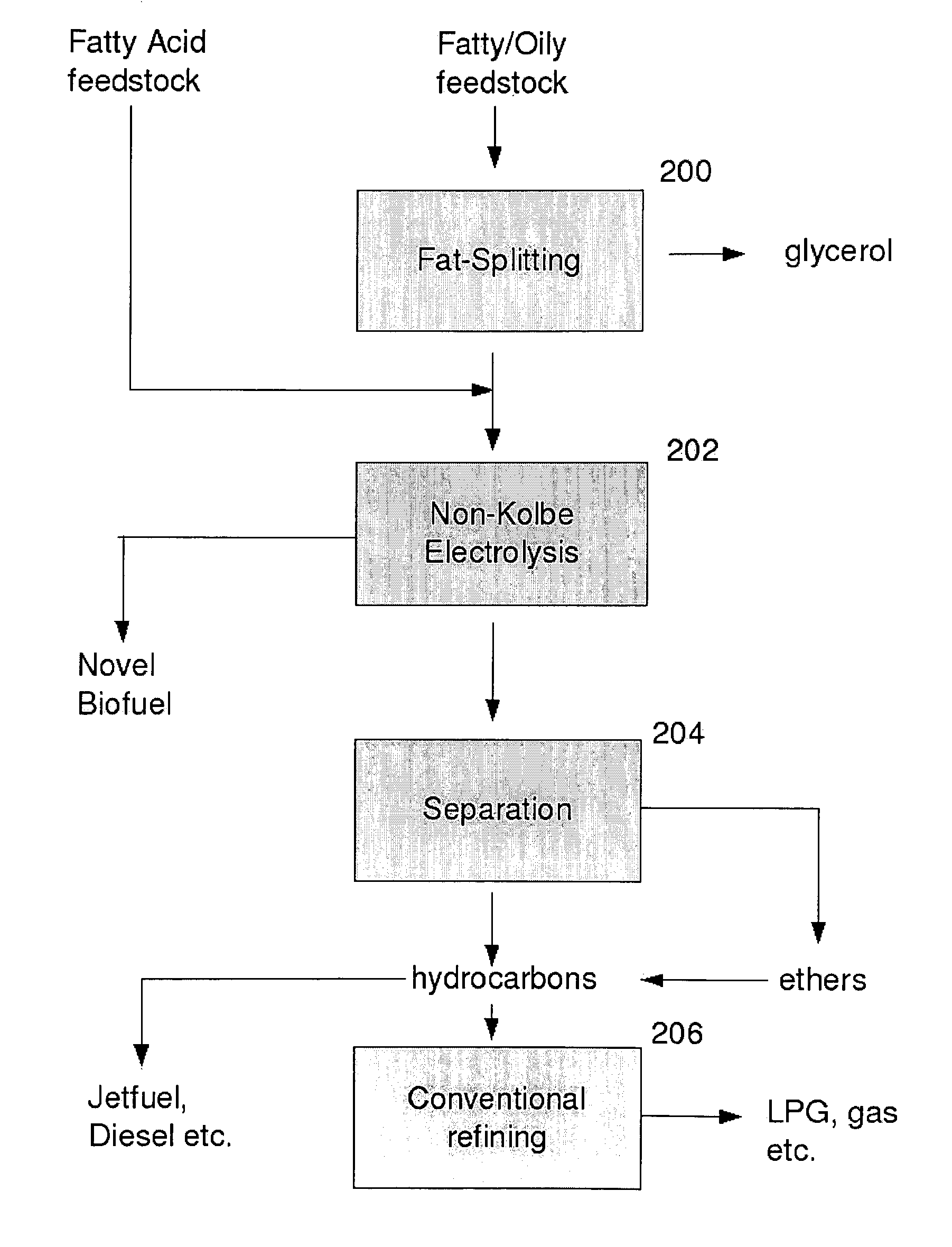
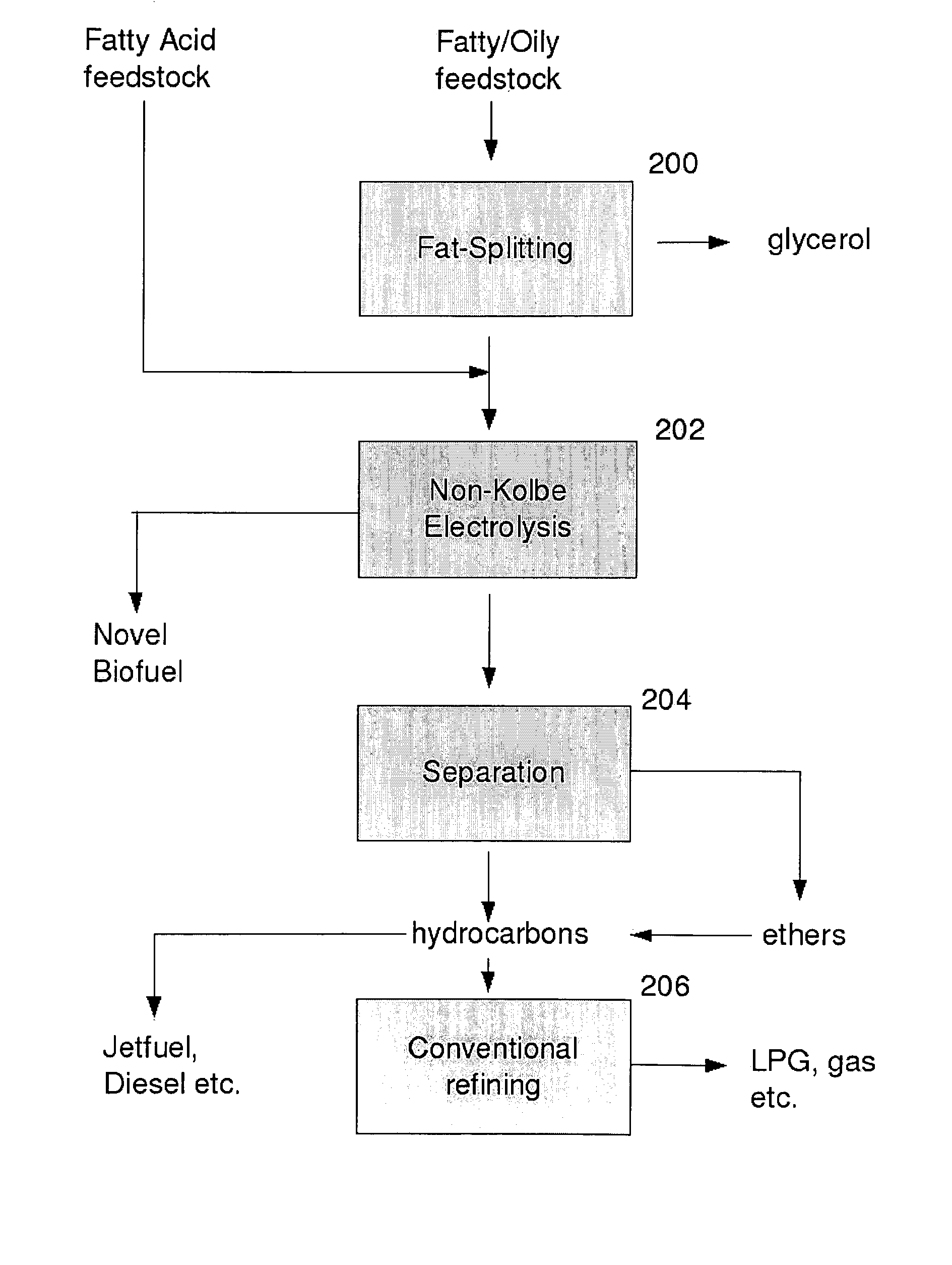
Biofuel composition and manufacturing process
InactiveUS20110035995A1Low melting pointReduce volatilityOrganic chemistryElectrolysis componentsAlcoholElectrolysis
A process for producing a fuel, which comprises the step of performing electrolysis on an alcoholic solution or a melt of a fatty acid or salt thereof or fatty acid ester or other derivative or precursor thereof, to decarboxylate said fatty acid or derivative, and produce a mixture of an ether and an alkene.
Owner:BUSCH RAINER
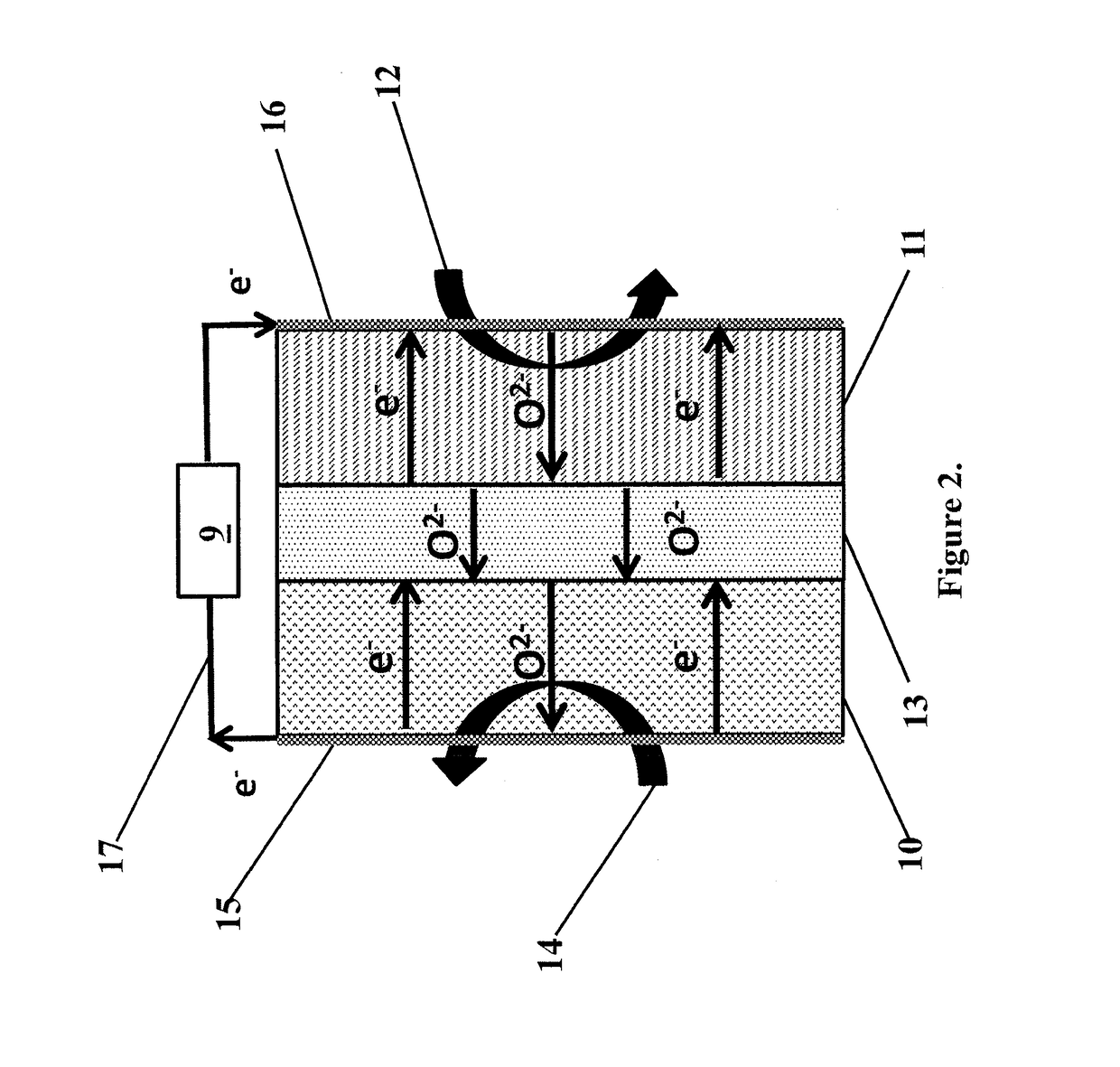
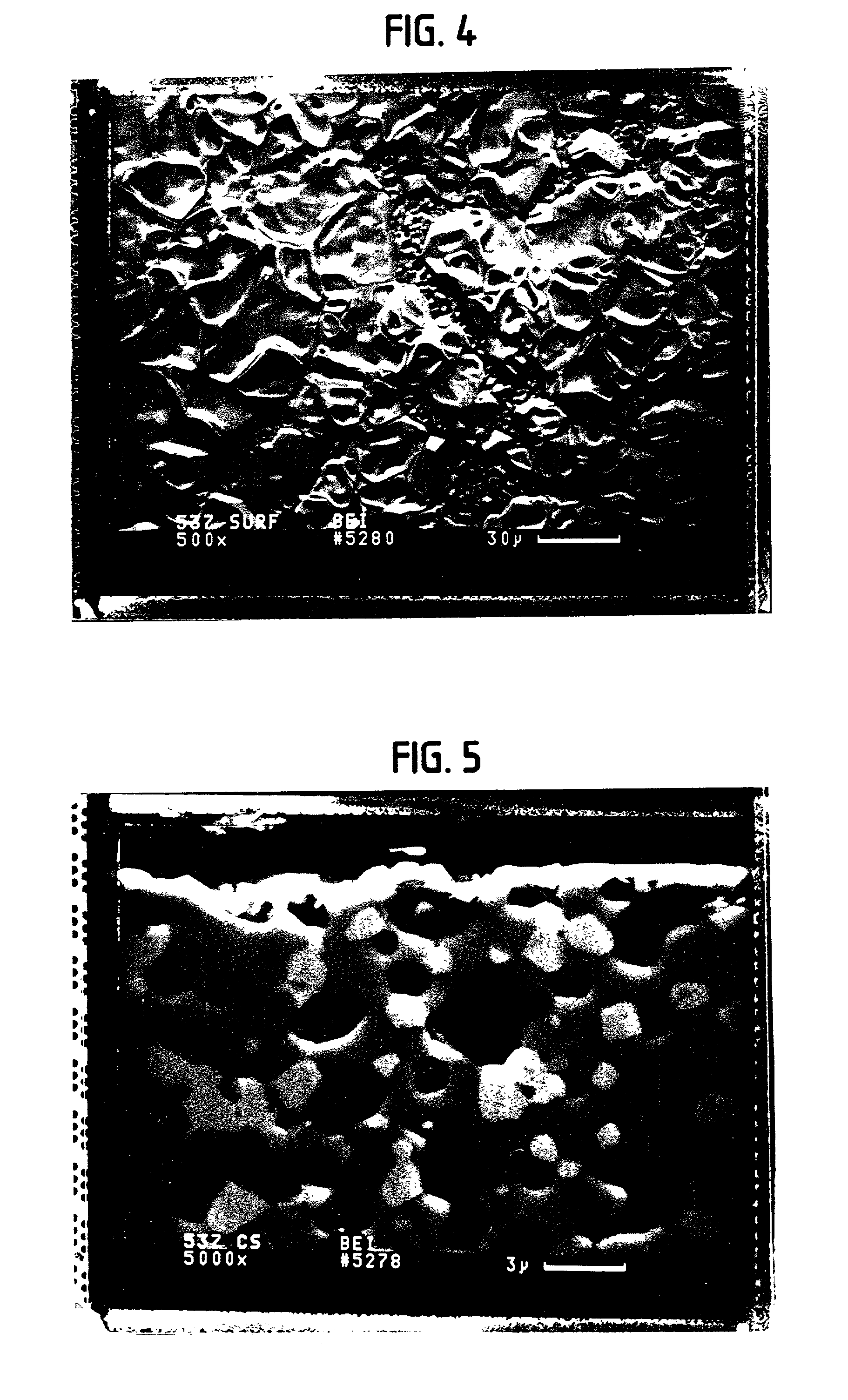
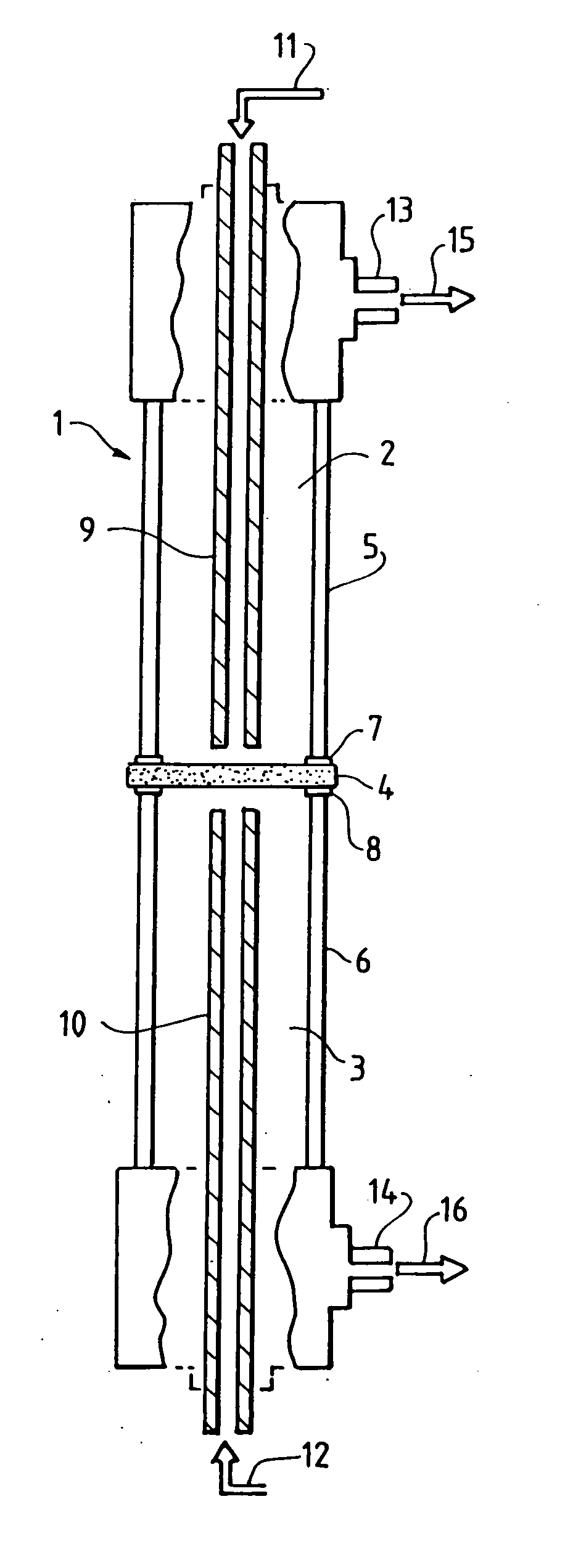
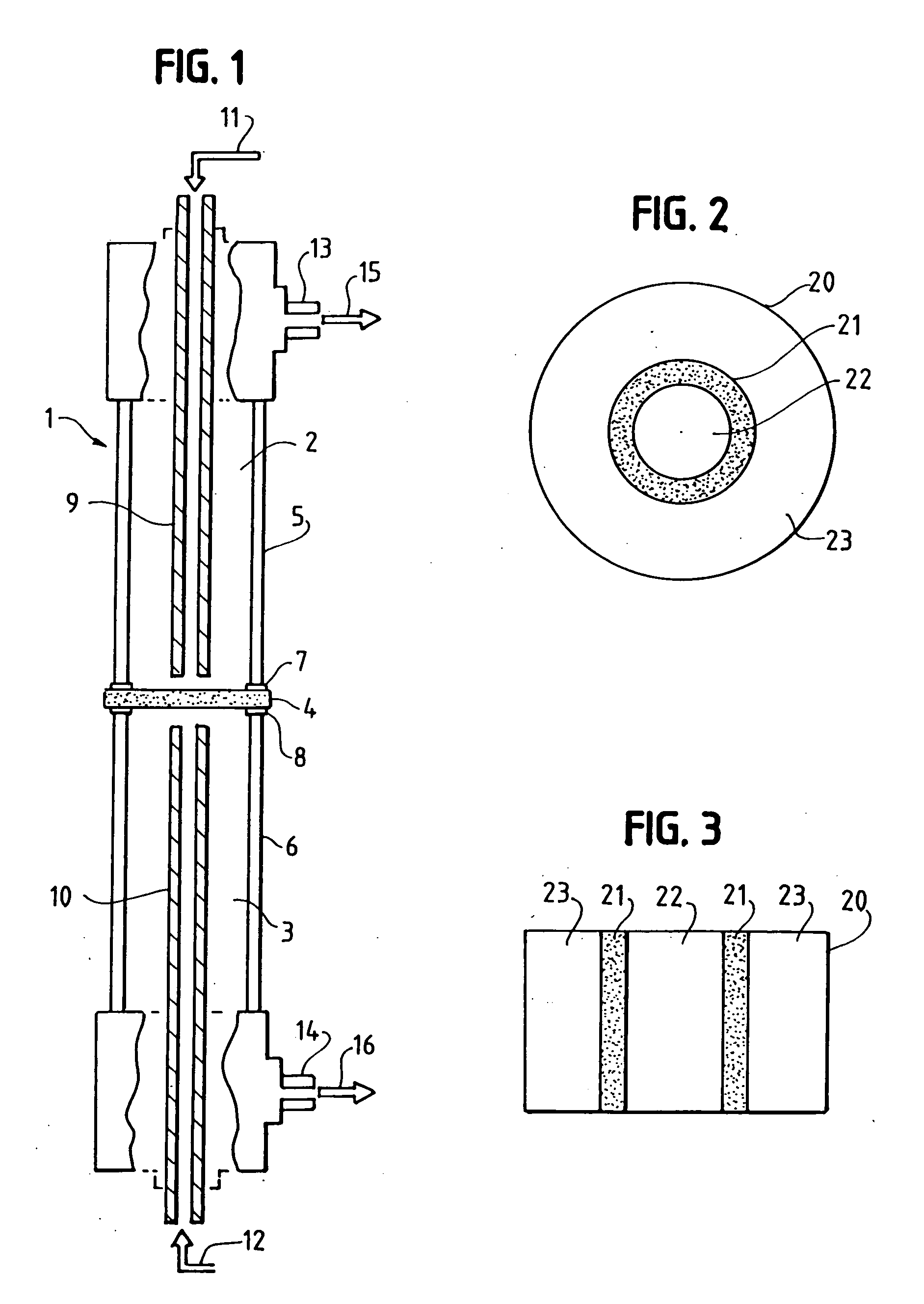
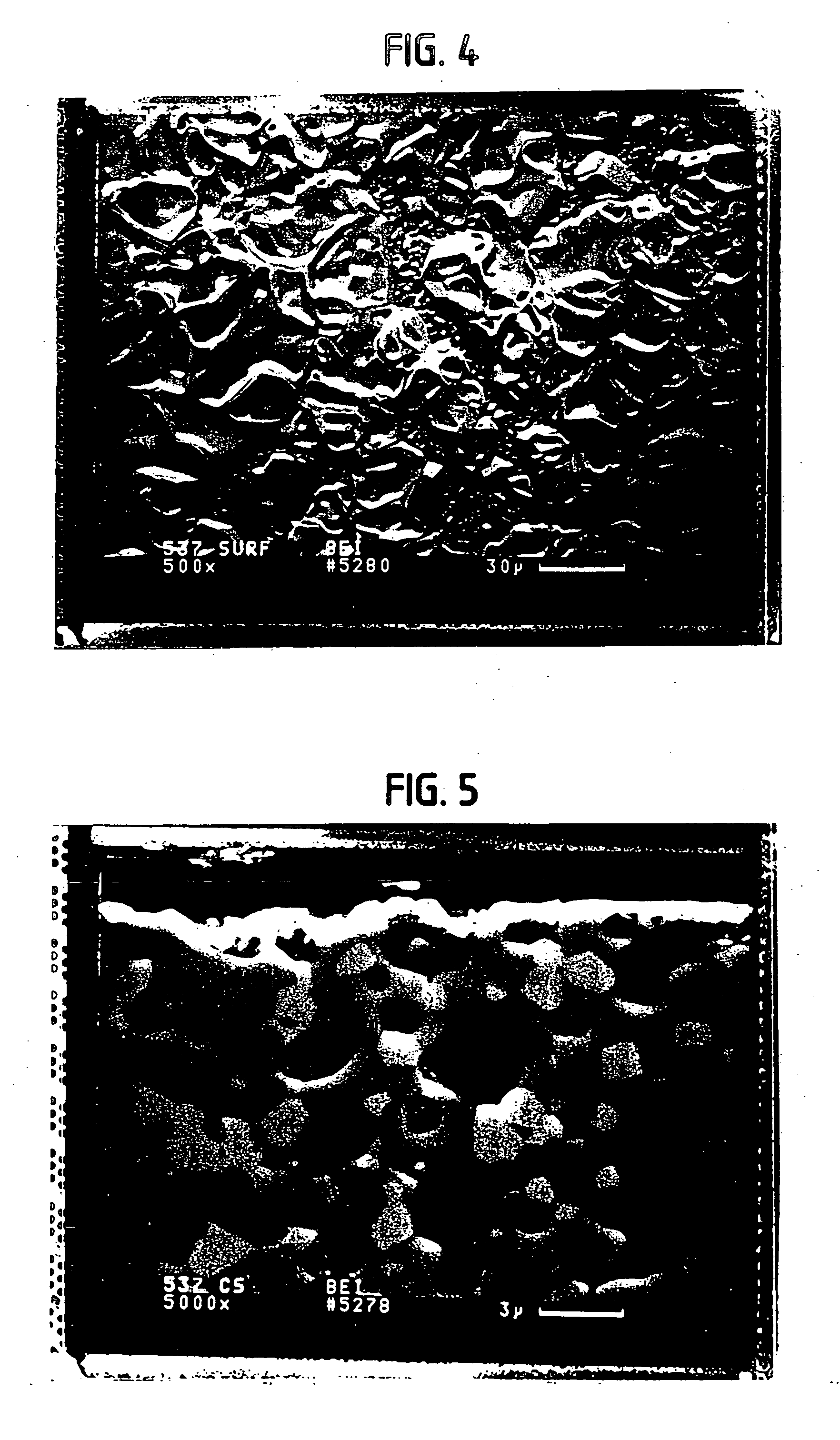
Solid multi-component membranes, electrochemical reactor components, electrochemical reactors and use of membranes, reactor components, and reactor for oxidation reactions
InactiveUS7033470B2Final product manufactureHydrogen/synthetic gas productionPorous substrateElectrochemical response
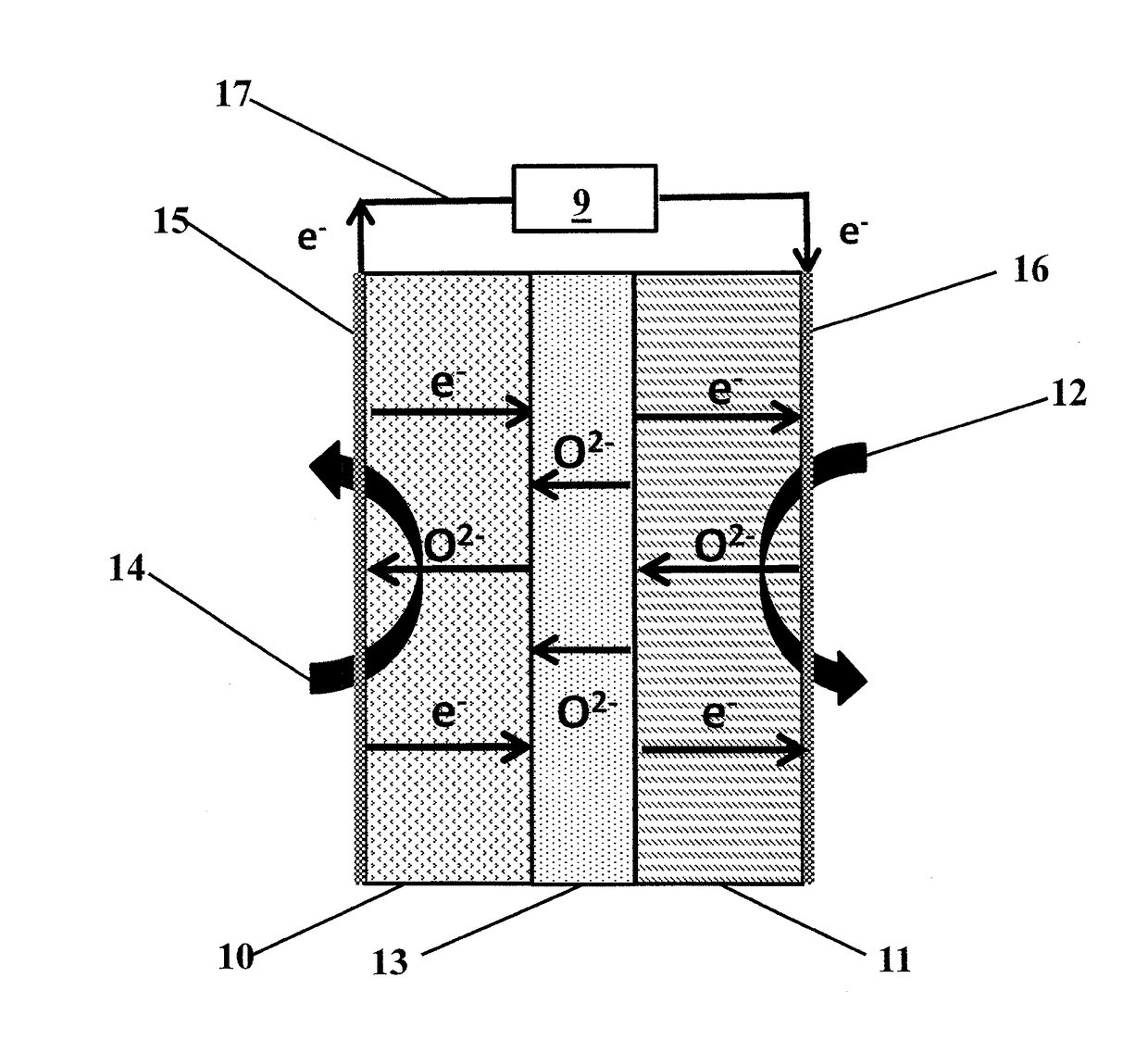
Solid membranes comprising an intimate, gas-impervious, multi-phase mixture of an electronically-conductive material and an oxygen ion-conductive material and / or a mixed metal oxide of a perovskite structure are described. Electrochemical reactor components, such as reactor cells, and electrochemical reactors are also described for transporting oxygen from any oxygen-containing gas to any gas or mixture of gases that consume oxygen. The reactor cells generally comprise first and second zones separated by an element having a first surface capable of reducing oxygen to oxygen ions, a second surface capable of reacting oxygen ions with an oxygen-consuming gas, an electron-conductive path between the first and second surfaces and an oxygen ion-conductive path between the first and second surfaces. The element may further comprise (1) a porous substrate, (2) an electron-conductive metal, metal oxide or mixture thereof and / or (3) a catalyst. The reactor cell may further comprise a catalyst in the zone which comprises a passageway from an entrance end to an exit end of the element. Processes described which may be conducted with the disclosed reactor cells and reactors include, for example, the partial oxidation of methane to produce unsaturated compounds or synthesis gas, the partial oxidation of ethanes substitution of aromatic compounds, extraction of oxygen from oxygen-containing gases, including oxidized gases, ammoxidation of methane, etc. The extraction of oxygen from oxidized gases may be used for flue or exhaust gas cleanup.
Owner:STANDARD OIL CO
Solid multi-component membranes, electrochemical reactor components, electrochemical reactors and use of membranes, reactor components, and reactor for oxidation reactions
InactiveUS20060131182A1Final product manufactureIron compoundsPorous substrateElectrochemical response
Solid membranes comprising an intimate, gas-impervious, multi-phase mixture of an electronically-conductive material and an oxygen ion-conductive material and / or a mixed metal oxide of a perovskite structure are described. Electrochemical reactor components, such as reactor cells, and electrochemical reactors are also described for transporting oxygen from any oxygen-containing gas to any gas or mixture of gases that consume oxygen. The reactor cells generally comprise first and second zones separated by an element having a first surface capable of reducing oxygen to oxygen ions, a second surface capable of reacting oxygen ions with an oxygen-consuming gas, an electron-conductive path between the first and second surfaces and an oxygen ion-conductive path between the first and second surfaces. The element may further comprise (1) a porous substrate, (2) an electron-conductive metal, metal oxide or mixture thereof and / or (3) a catalyst. The reactor cell may further comprise a catalyst in the zone which comprises a passageway from an entrance end to an exit end of the element. Processes described which may be conducted with the disclosed reactor cells and reactors include, for example, the partial oxidation of methane to produce unsaturated compounds or synthesis gas, the partial oxidation of ethane, substitution of aromatic compounds, extraction of oxygen from oxygen-containing gases including oxidized gases, ammoxidation of methane, etc. The extraction of oxygen from oxidized gases may be used for flue or exhaust gas cleanup.
Owner:MAZANEC TERRY J +3
Method for the production of olefins, an olefin, a polyolefin, and use of the polyolefin
InactiveUS20130203953A1Maximize formationLow costElectrolysis componentsOrganic chemistryPolyolefinPropanoic acid
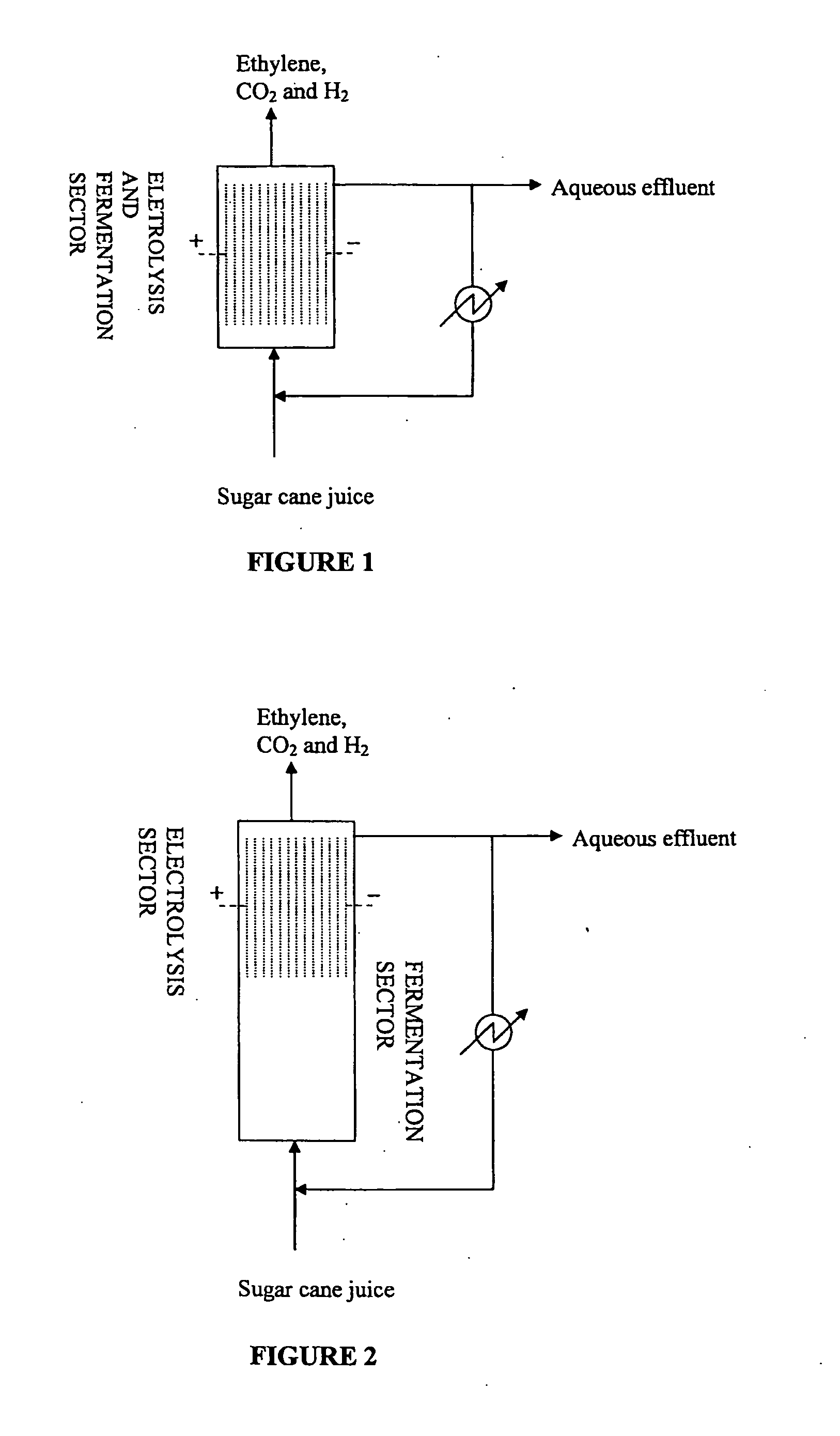
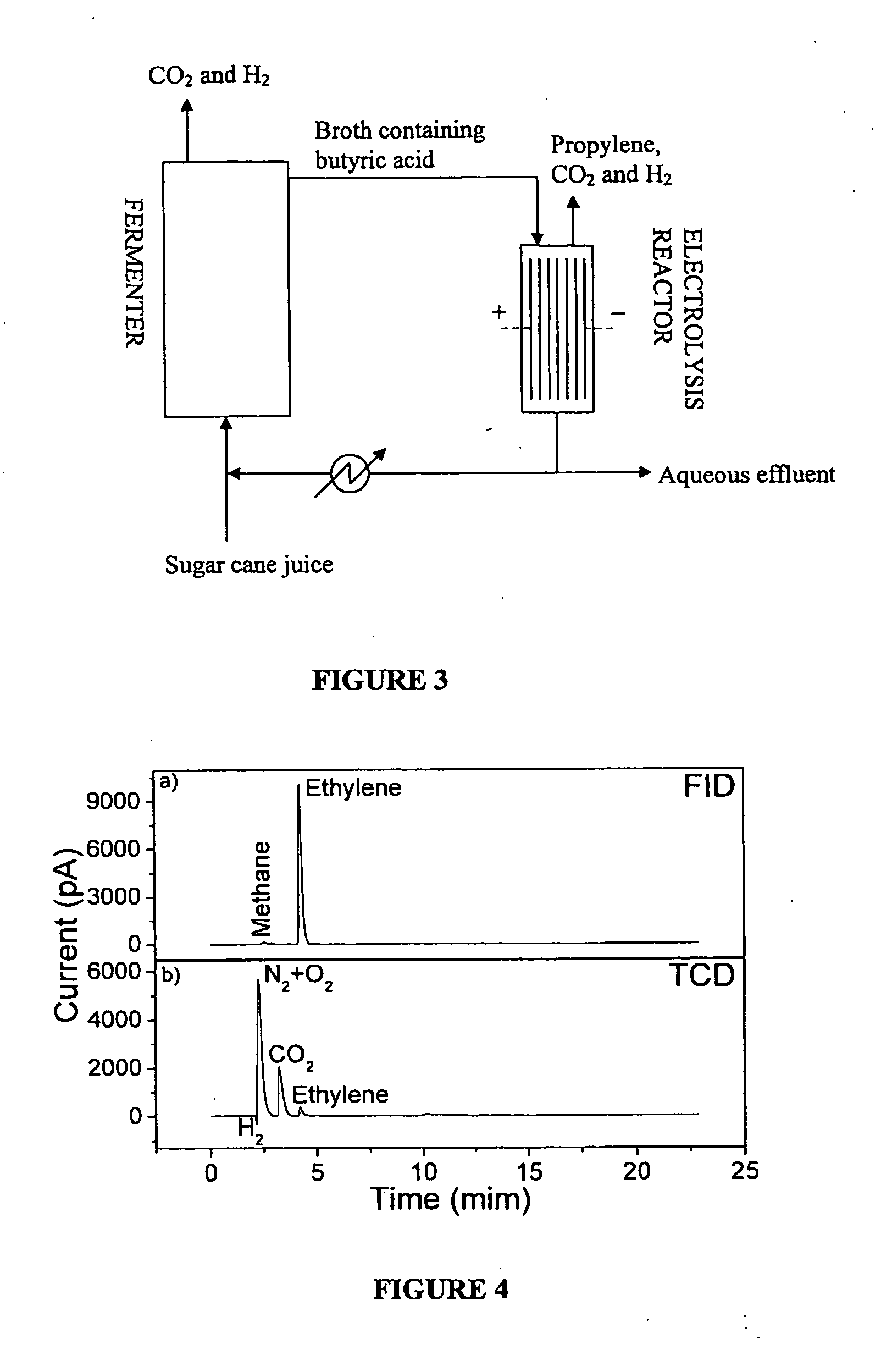
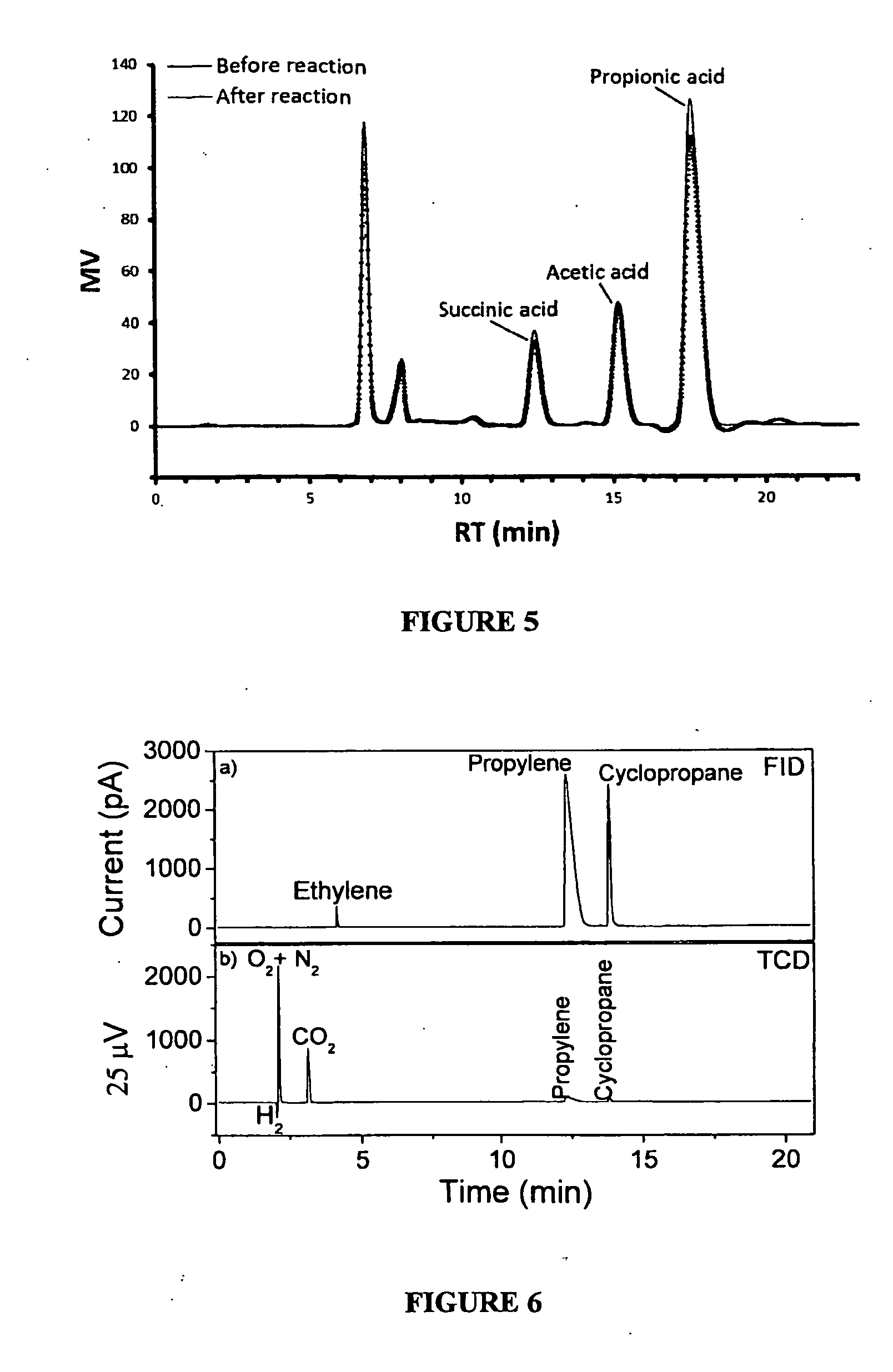
The present invention relates to a method for the production of an olefin from at least one renewable natural raw material. More specifically, the present invention refers to a method whereby is obtained ethylene or propylene at high yield and high productivity by means of the anodic electrodecarboxylation reaction of carboxylic acids, respectively propionic acid and butyric acid, produced from fermentation, preferably of sugars. The method for generating the olefin is simple, has a low cost, and provides low emissions of greenhouse gasses of fossil origin.
Owner:BRASKEM SA +1
Metal oxide-organic hybrid materials for heterogeneous catalysis and methods of making and using thereof
ActiveUS20160152648A1Improve robustnessPreparation by oxidation reactionsPlatinum group organic compoundsElectrolysisCobalt
Owner:YALE UNIV
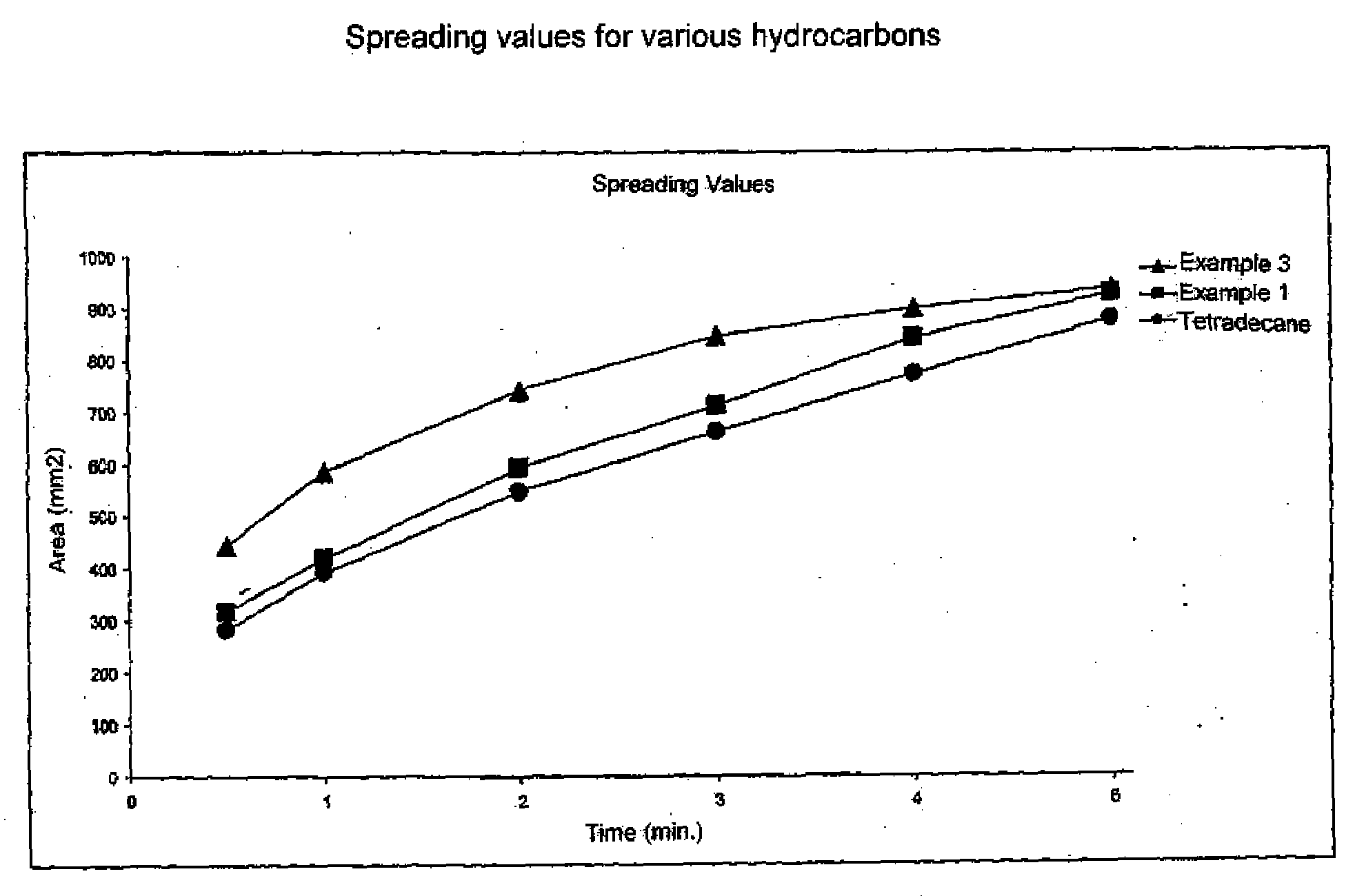
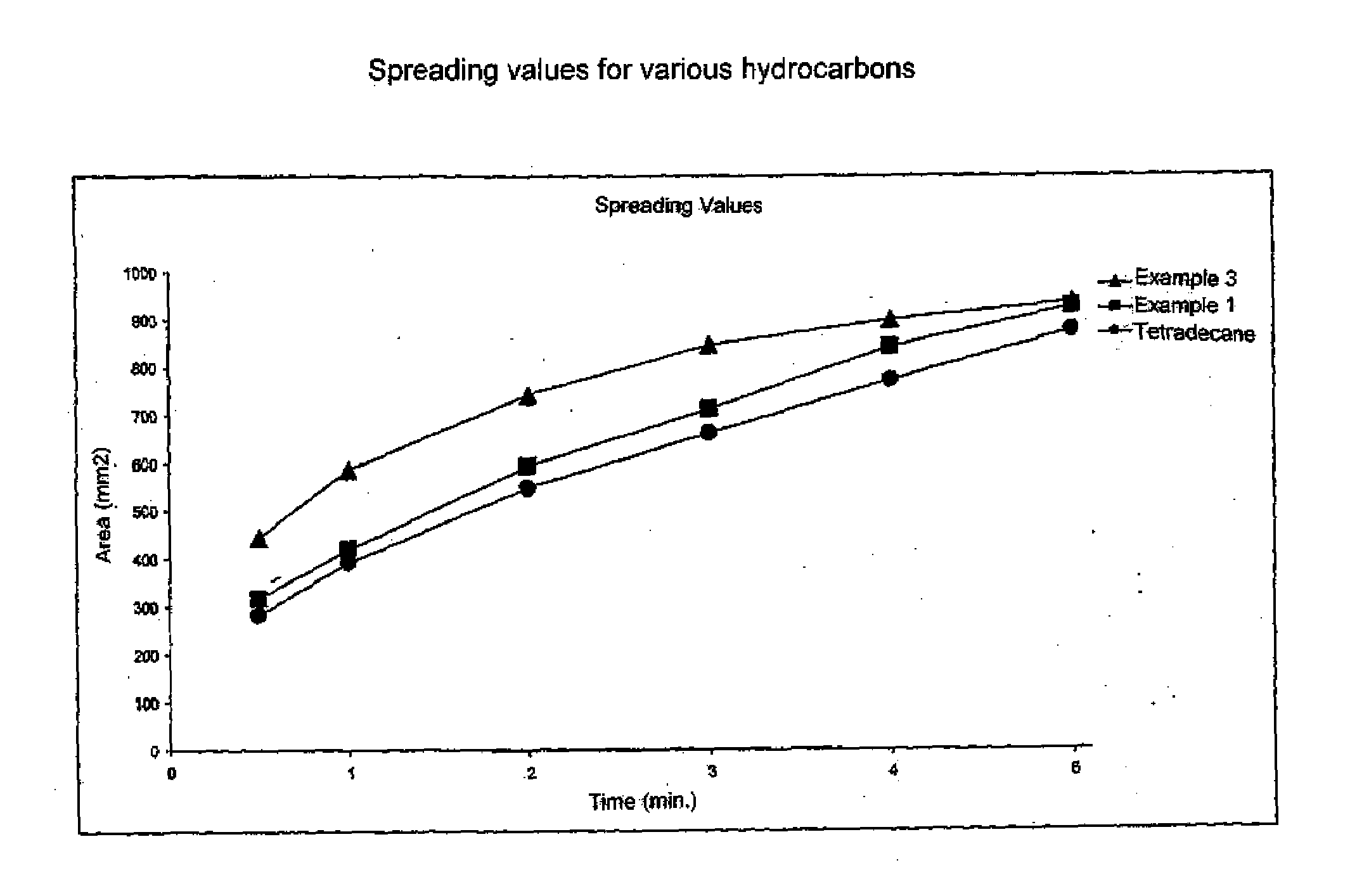
Emollients and Cosmetic Compositons Based on Specific Branched Hydrocarbons
InactiveUS20080161418A1Improve spreading qualityIncrease productionBiocideCosmetic preparationsBranched chain fatty acidsHydrocarbon
A cosmetic or pharmaceutical composition is provided. The composition contains a mixture of oils prepared by Kolbe electrolysis of branched chain fatty acids and mixtures of branched chain fatty acids with straight chain fatty acids. The fatty acids containing from 3 to 26 carbon atoms. The oils are a mixture of oils with different spreading rates (spreading cascade).
Owner:COGNIS IP MANAGEMENT GMBH
Method of exfoliating and functionalizing graphite anode
A. providing an electrochemical cell with a first graphitic electrode and a second conductive electrode, wherein the first graphitic electrode is made of any one of HOPG, natural graphite, and synthetic graphite, the first graphitic electrode is held at a most positive potential, and the second conductive electrode is conductive, wherein a current passes through the electrochemical cell; B. providing an electrolyte of a solvent in the electrochemical cell, wherein the electrolyte has specific oxygen containing salts and base. Thereby, the graphitic electrode is functionalized and exfoliated by applying a voltage between the two electrodes thus producing graphene oxide.
Owner:LAI CHUNG PING
Methods for the electroreduction of carbon dioxide to value added chemicals
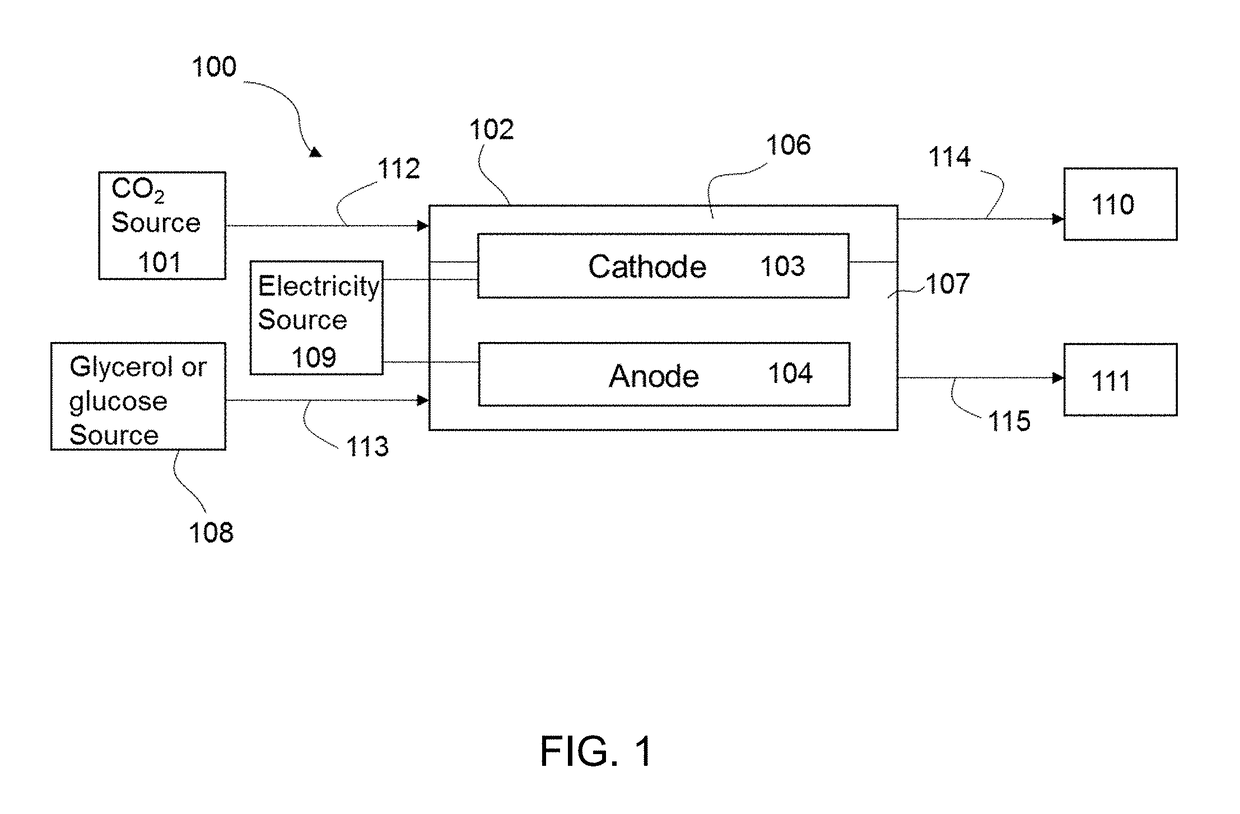
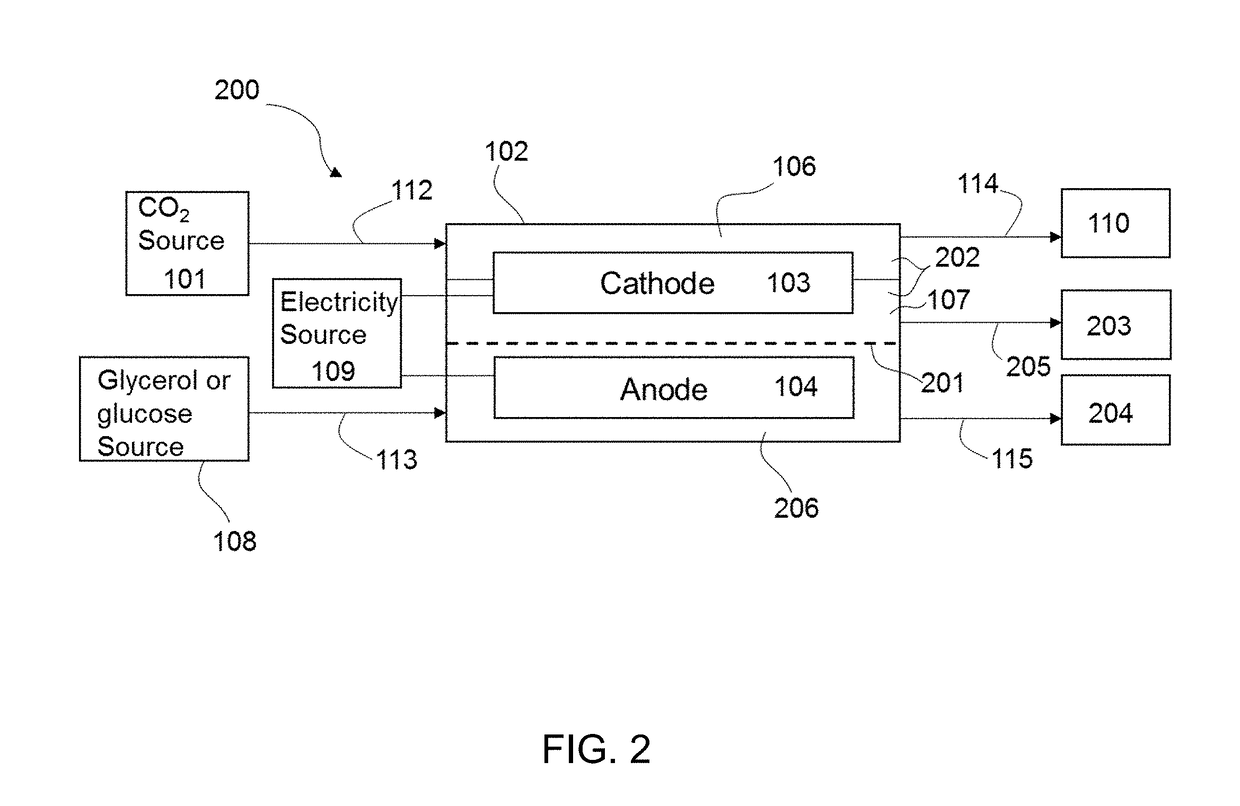
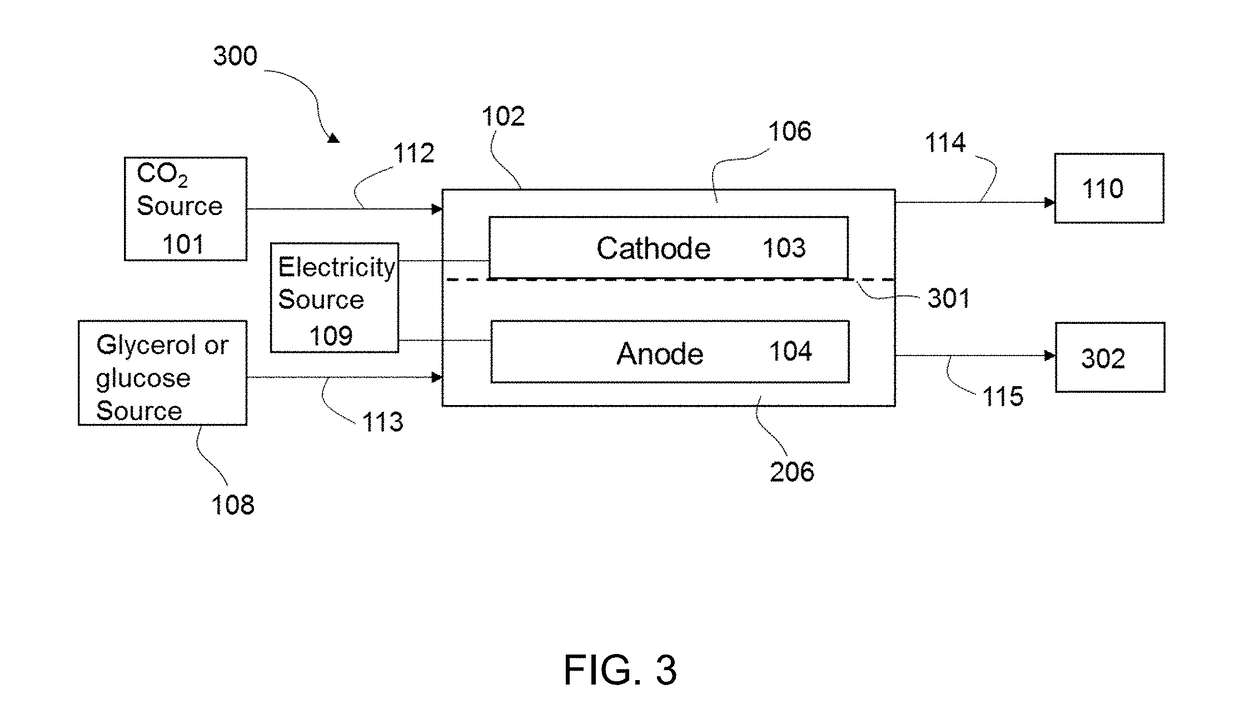
InactiveUS20190055656A1Reduce carbon dioxideReduce the carbon dioxideCellsElectrogenerative processesElectricityGlycerol
The present disclosure provides a method of electroreducing carbon dioxide (CO2). The method of electroreducing carbon dioxide may include feeding a first stream comprising carbon dioxide into a chamber through a chamber inlet, the chamber containing a gas diffusion cathode and a gas diffusion anode; feeding a second stream comprising glycerol or glucose into the chamber, the second stream having a pH of 12 to 14; and applying an electrical potential between the gas diffusion anode and the gas diffusion cathode to reduce the carbon dioxide and oxidize the glycerol or glucose.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Production of fuel from chemicals derived from biomass
Hydrocarbons may be formed from six carbon sugars. This process involves obtaining a quantity of a hexose sugar. The hexose sugar may be derived from biomass. The hexose sugar is reacted to form an alkali metal levulinate, an alkali metal valerate, an alkali metal 5-hydroxy pentanoate, or an alkali metal 5-alkoxy pentanoate. An anolyte is then prepared for use in a electrolytic cell. The anolyte contains the alkali metal levulinate, the alkali metal valerate, the alkali metal 5-hydroxy pentanoate, or the alkali metal 5-alkoxy pentanoate. The anolyte is then decarboxylated. This decarboxylating operates to decarboxylate the alkali metal levulinate, the alkali metal valerate, the alkali metal 5-hydroxy pentanoate, or the alkali metal 5-alkoxy pentanoate to form radicals, wherein the radicals react to form a hydrocarbon fuel compound.
Owner:ENLIGHTEN INNOVATIONS INC
Iridium complexes for electrocatalysis
ActiveUS9790605B2Improve stabilityConvenient bindingIndium organic compoundsCell electrodesIridiumPentamethylcyclopentadiene
Solution-phase (e.g., homogeneous) or surface-immobilized (e.g., heterogeneous) electrode-driven oxidation catalysts based on iridium coordination compounds which self-assemble upon chemical or electrochemical oxidation of suitable precursors and methods of making and using thereof are. Iridium species such as {[Ir(LX)x(H2O)y(μ-O)]zm+}n wherein x, y, m are integers from 0-4, z and n from 1-4 and LX is an oxidation-resistant chelate ligand or ligands, such as such as 2(2-pyridyl)-2-propanolate, form upon oxidation of various molecular iridium complexes, for instance [Cp*Ir(LX)OH] or [(cod)Ir(LX)] (Cp*=pentamethylcyclopentadienyl, cod=cis-cis,1,5-cyclooctadiene) when exposed to oxidative conditions, such as sodium periodate (NaIO4) in aqueous solution at ambient conditions.
Owner:YALE UNIV
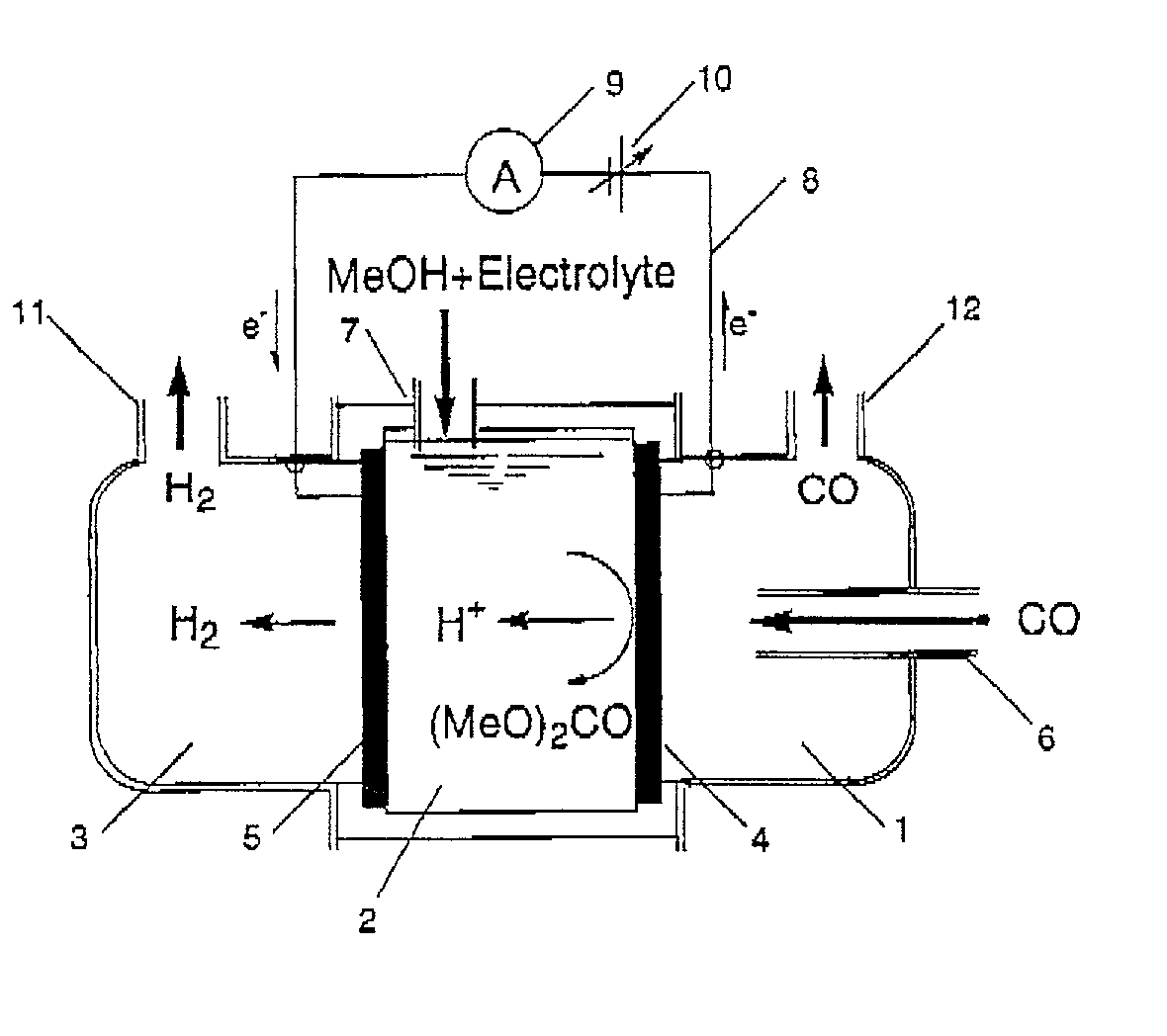
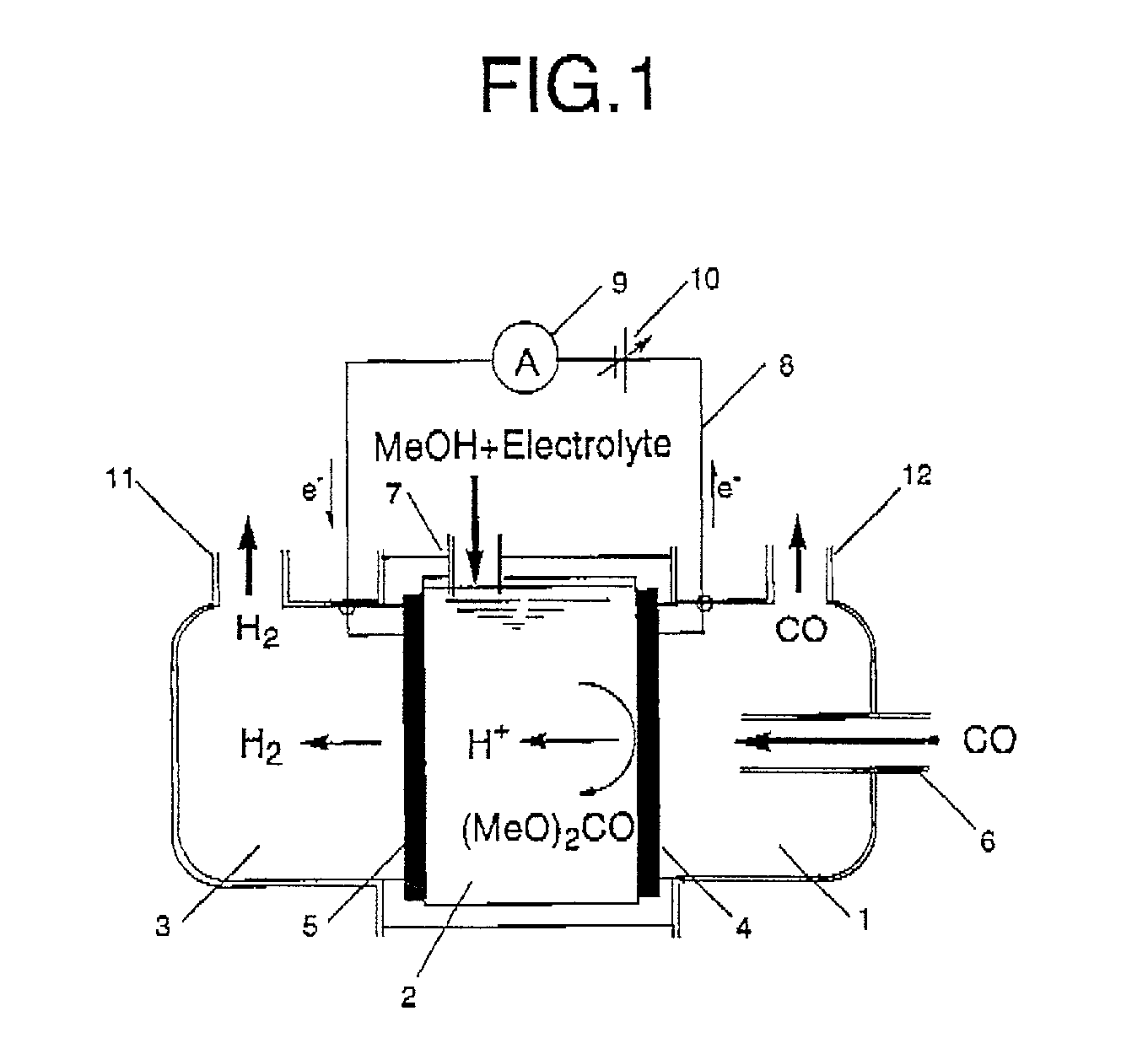
Synthetic method and synthetic system
Provided is a synthesis method comprising a first step of producing a carbonate compound from carbon monoxide and an alcohol-based compound at an anode of a first electrochemical cell comprising a cathode and the anode, and a second step of synthesizing a first product by a dealcoholization reaction of the carbonate compound, wherein an alcohol-based compound eliminated in the second step is recycled in the first step.
Owner:SEKISUI CHEM CO LTD
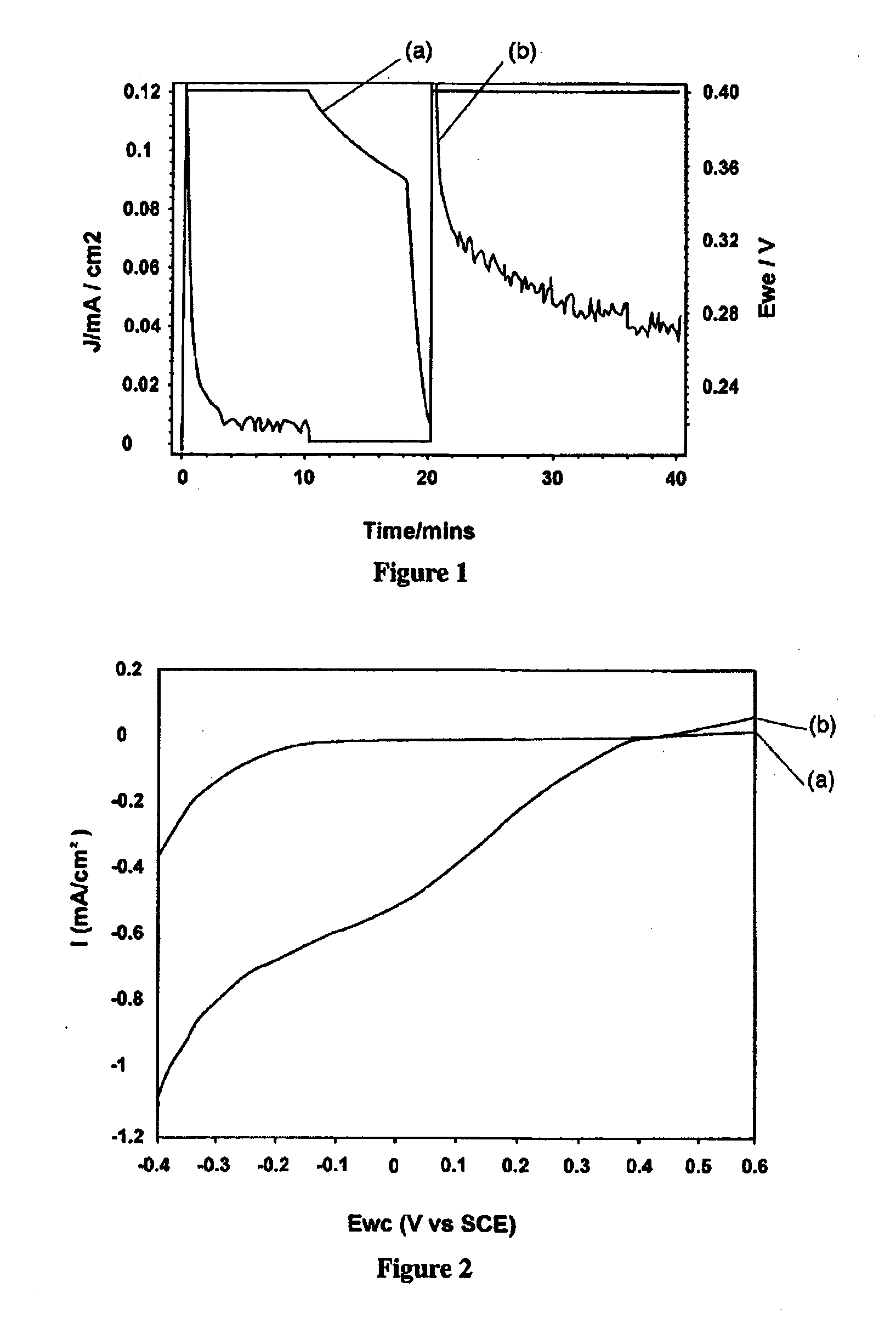
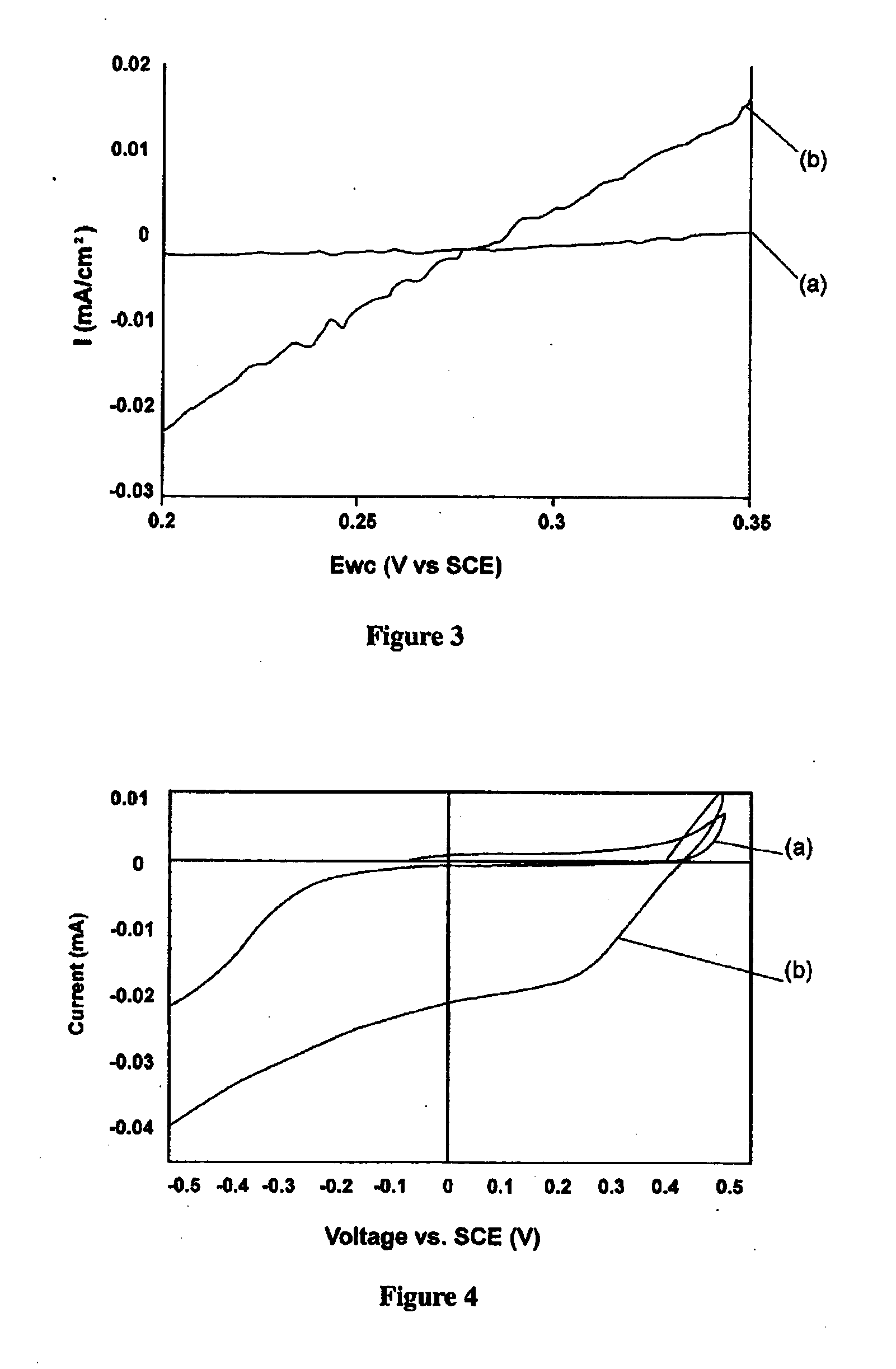
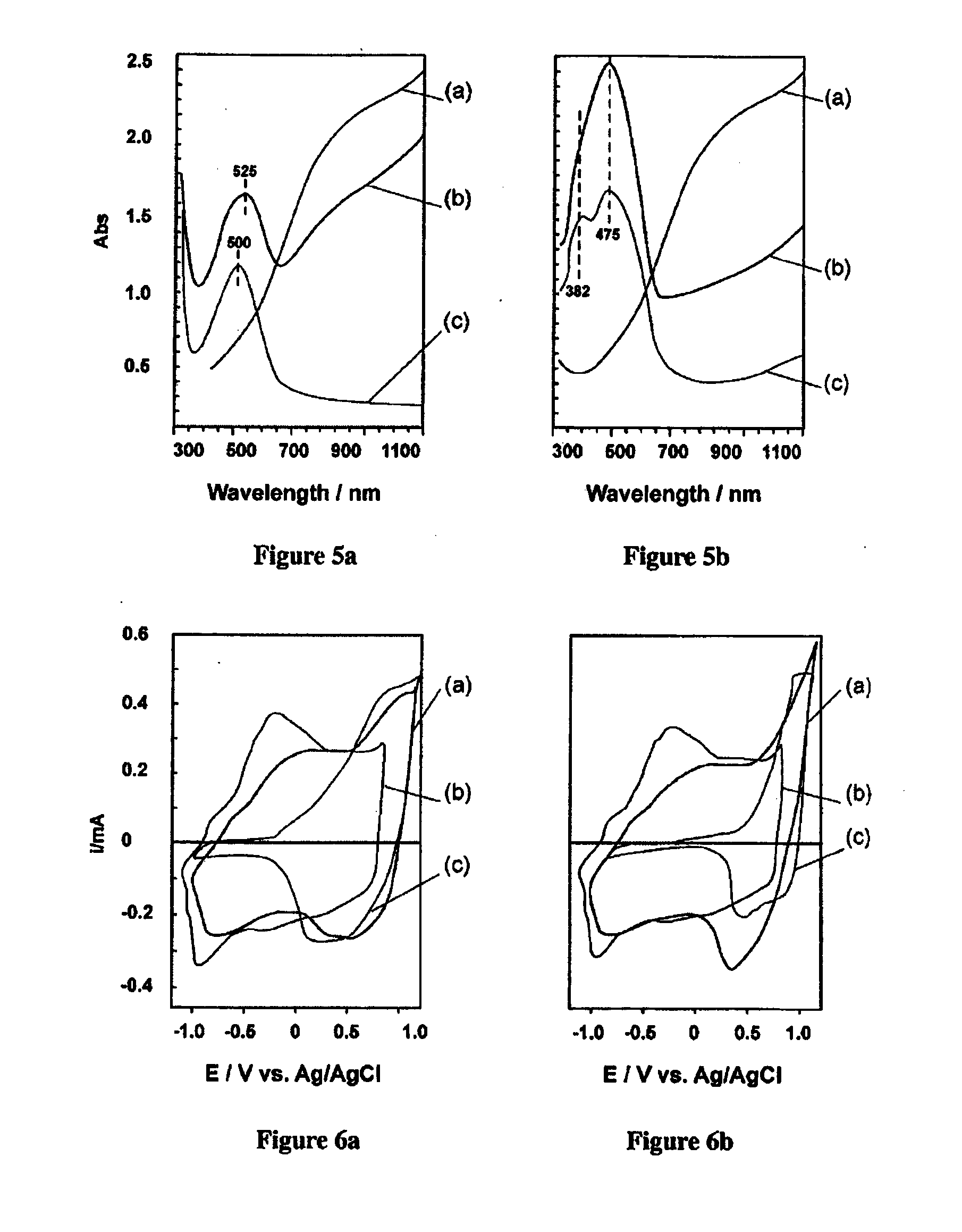
Process for the production of diaryl iodonium compounds
InactiveUS20050145504A1Easy to convertEliminate riskElectrolysis componentsElectrolytic organic oxidationArylIodine
The present invention provides an electrochemical method for producing diaryl iodonium compounds wherein application of an electric current to an electrochemical cell containing a reaction mixture composed of a solvent, an iodoaryl compound and an electrolyte forms an oxidizing agent in situ. In this first step, the oxidizing agent is subsequently converted into a stable oxidized iodoaryl intermediate, typically an iodosyl compound. The electric potential is removed and in a second step a target aryl compound is introduced to the reaction mixture to react with the oxidized iodoaryl intermediate to form a diaryl iodonium compound.
Owner:CORNELL DEVMENT
Method and system for catalysis
InactiveUS20130220822A1Facilitates charge charge separationEasy to transportMachining electrodesPhotography auxillary processesElectricityOxidation-Reduction Agent
A catalyst comprising a first conjugated polymer material that forms an interface with a second material, wherein charge is separated from photo excited species generated in one or both of the first and second materials and subsequently participates in a reaction, electro-catalytic reactions or redox reactions.
Owner:AQUAHYDREX INC
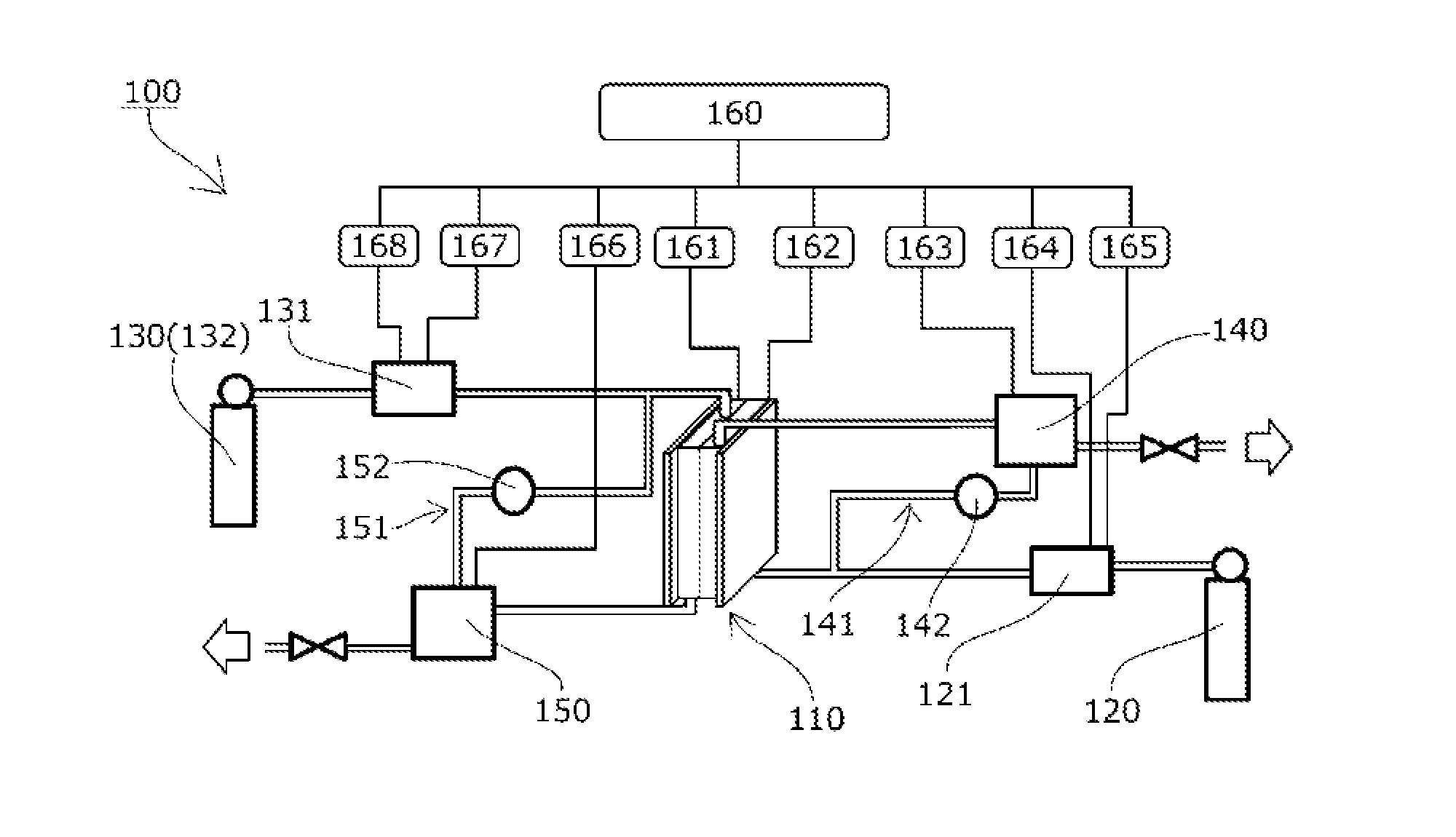
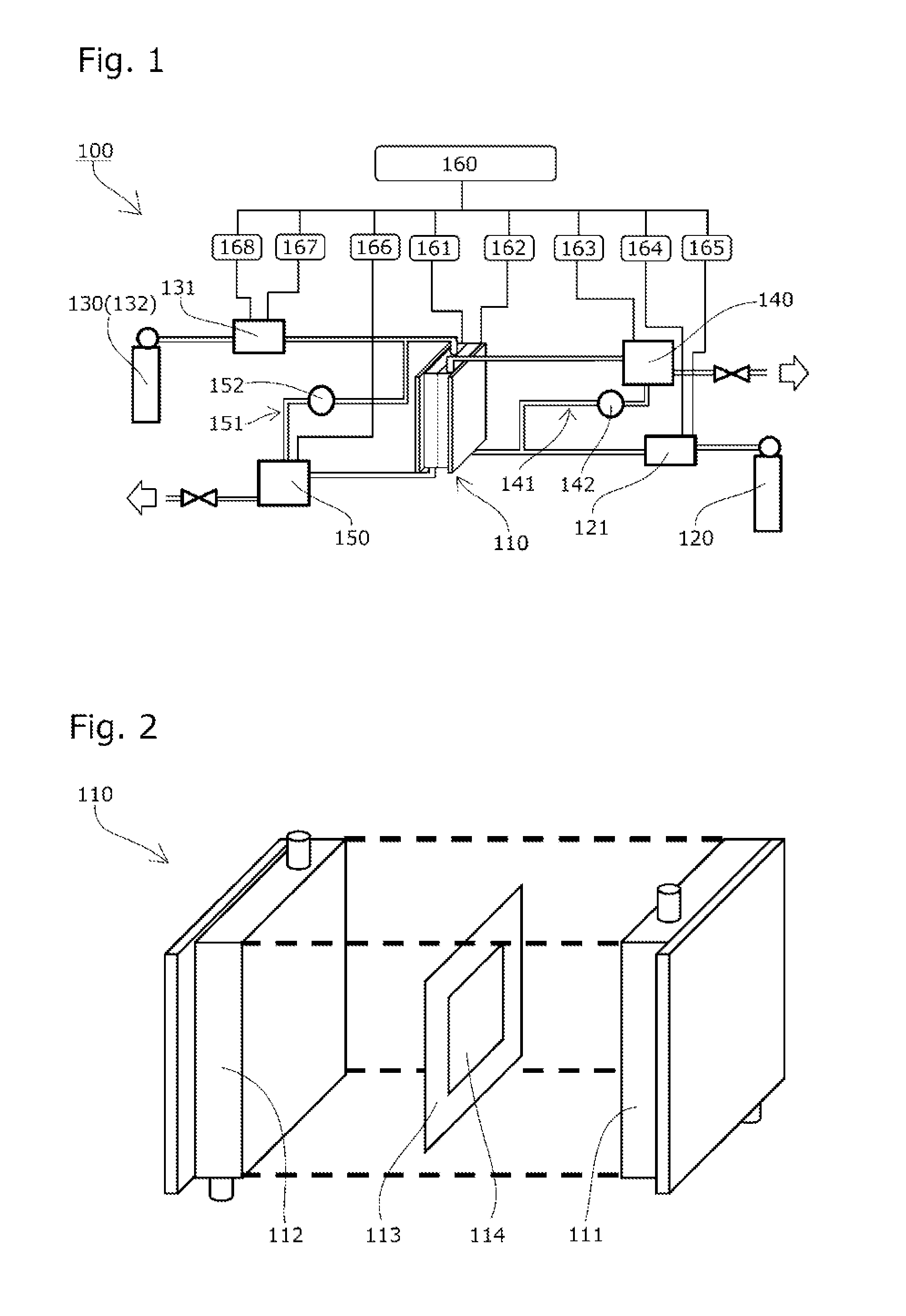
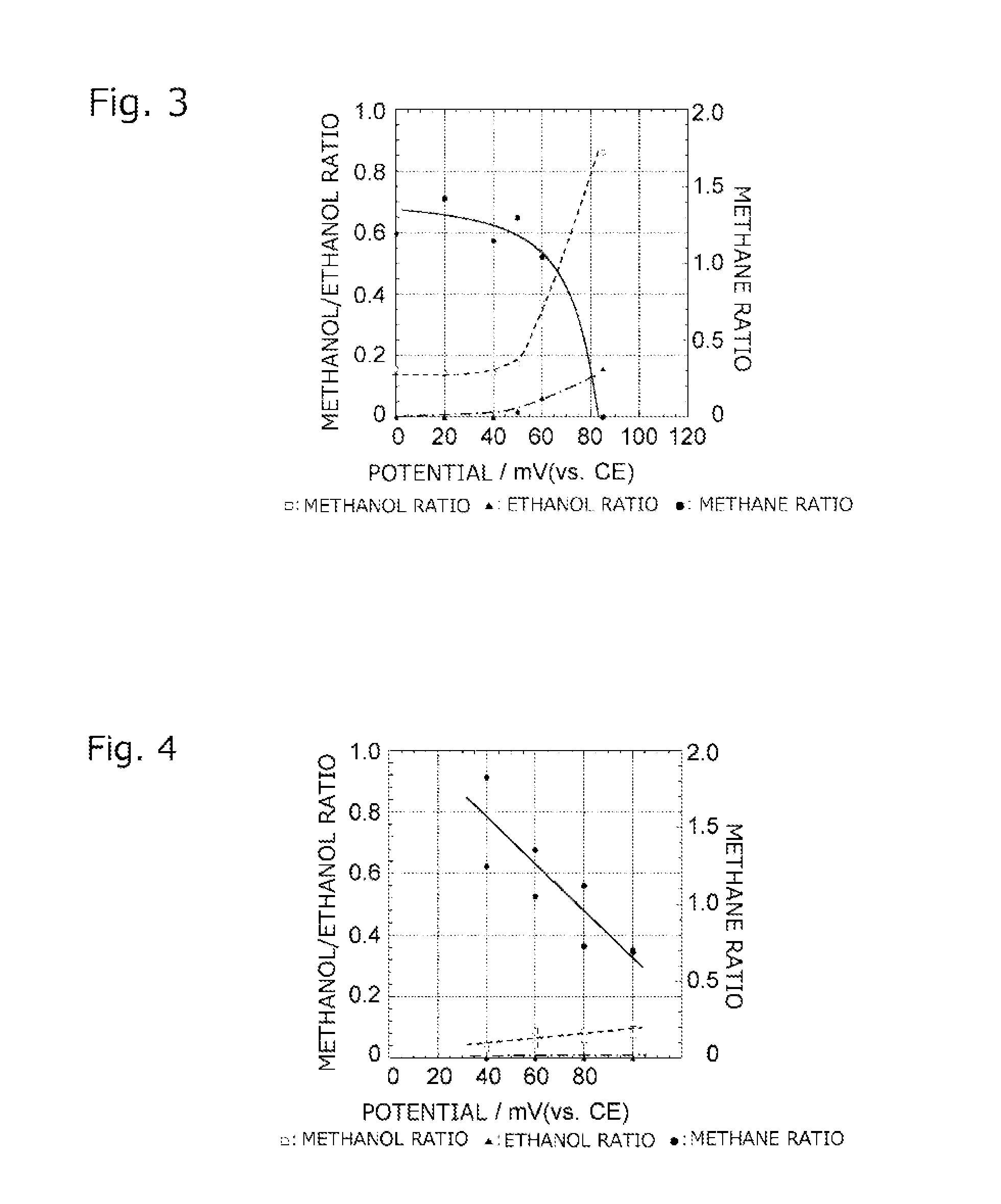
Solid polymer power generation or electrolysis method and system
ActiveUS20160226090A1Reduce carbon dioxideImprove utilization efficiencyCellsSolid electrolytesElectrolysisHydrogen supply
There are provided: a solid polymer power generation or electrolysis method that does not require injection of energy from the outside and maintenance of a high temperature, and is capable of converting carbon dioxide to a useful hydrocarbon while producing energy, controlling the production amounts of the hydrocarbons or the like and a ratio sorted by kind of the hydrocarbons, improving utilization efficiency of a product, and simplifying equipment for separation and recovery; and a system for implementing the solid polymer power generation or electrolysis method. Carbon dioxide is supplied to the side of one electrode 111 of a reactor 110 having a membrane electrode assembly 113, hydrogen is supplied to the side of the other electrode 112, and the amounts of the hydrocarbons produced per unit time and the ratio sorted by kind of the hydrocarbons are changed by controlling a power generation voltage of the reactor 110.
Owner:JAPAN AEROSPACE EXPLORATION AGENCY
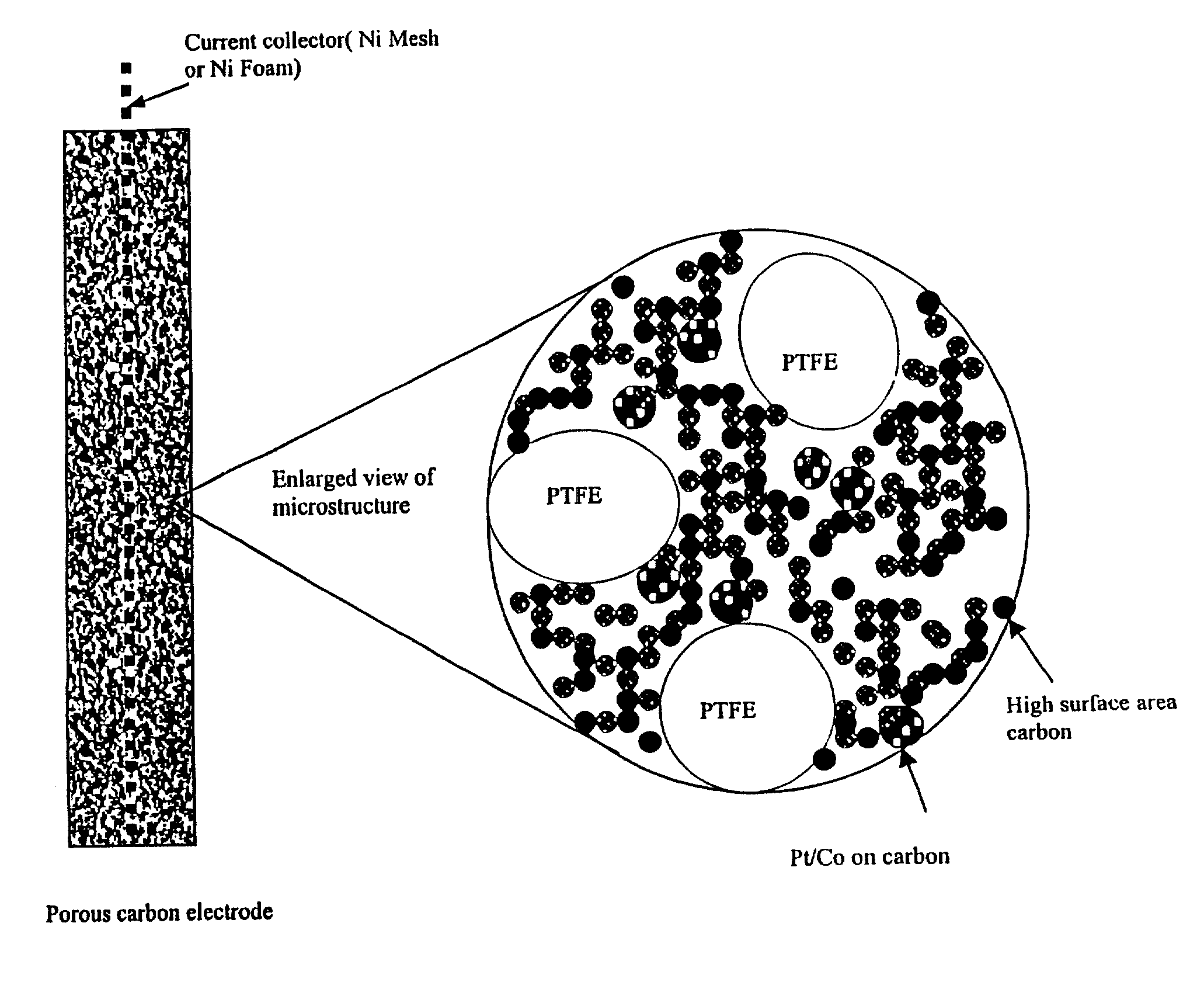
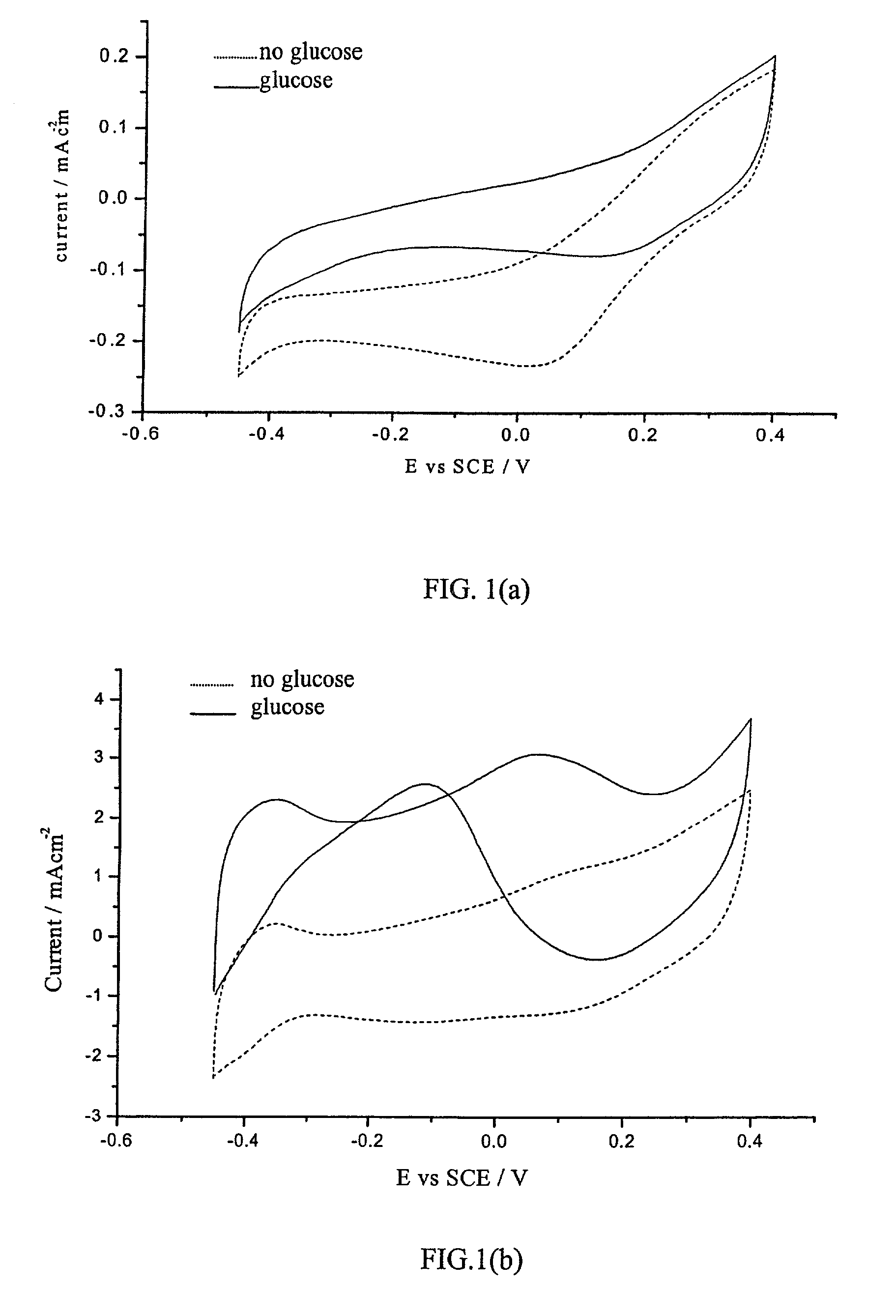
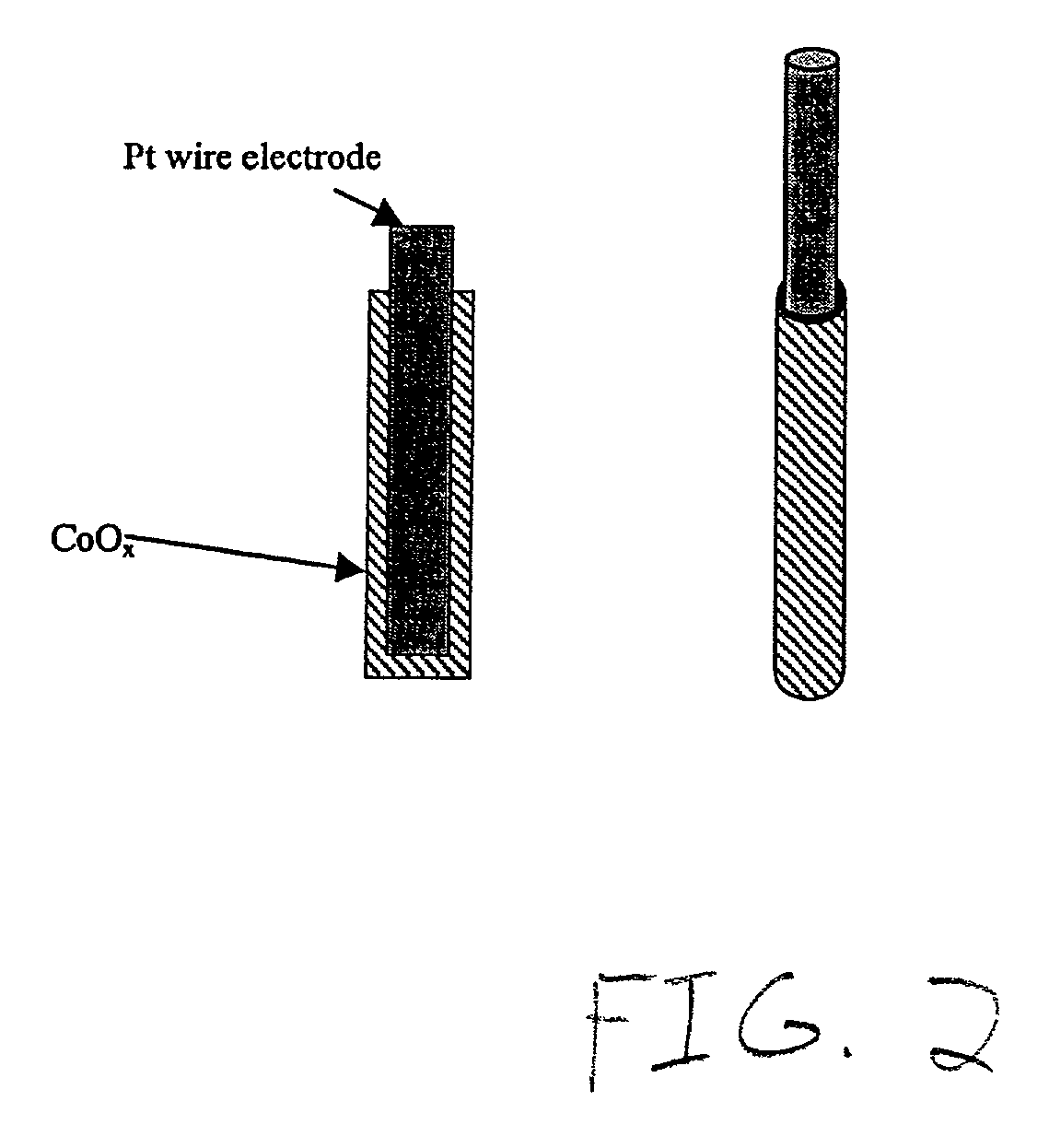
Methods and apparatus for the oxidation of glucose molecules
InactiveUS7419580B2Moderate to high current densityReduce oxidationImmobilised enzymesCell electrodesGlucose sensorsConcentrations glucose
A catalyst comprising Pt—Co alloy, or Pt—Co—Sn alloy or Pt—ComOn mixed metal oxides is disclosed to be used as a catalyst for the direct electrochemical oxidation of glucose or other simple sugars and carbohydrates at room temperature. The catalyst can be supported on metal electrodes, graphite electrodes, porous carbon electrodes, or gas diffusion electrodes. An electrode containing this catalyst will be used as the key component in a direct glucose-air fuel cell operating in alkaline media with a good room temperature performance. This catalyst can also be applied as a key electrode material in a glucose sensor to detect glucose concentration in neutral or alkaline medium. The preparation method of the catalyst, optimum composition, and results of glucose sensor and glucose fuel cell applications are disclosed.
Owner:UNIV OF HONG KONG RES CONSULTING SHENZHEN
Method, container and uses for converting biomass materials into soluble substances by one-step
InactiveUS20170107632A1Reduce materialImprove efficiencyCellsElectrode shape/formsElectrolysisBiomass
The present invention discloses a method for converting biomass material into soluble substances by one-step, comprising: converting biomass materials into soluble substances by one-step by electrolyzing at a certain time under the condition of constant current using bipolar three-dimension-electrodes system, wherein bipolar three-dimension-electrodes system including an anode, a cathode, particle electrodes and electrolyte, and in electrolyzing process, the particle electrodes and the biomass materials being suspended in the electrolyte. The present invention also discloses a container for converting biomass materials into soluble substances by one-step, comprising: A tank which holds electrolyte at the interior thereof, wherein the side wall of the tank is provided with an opening, the opening is provided with a permeable membrane, and the opening also communicates with a discharge pipe; A pair of electrode plates which are immersed into the electrolyte by at least a part thereof, wherein the pair of electrode plates space from each other and connect to positive pole and negative pole of power supply respectively to form anode and cathode in energized state respectively; and, Particle electrodes which are in granular form and are suspended in the electrolyte. The present invention also discloses uses of soluble substances that are prepared by any one of the methods.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI TIANJIN CHINA
Enantiospecific catalysts prepared by chiral deposition
InactiveUS20050045489A1Easy to separateSimple and inexpensiveElectrolysis componentsElectrolytic inorganic material coatingSolid substrateMetal
Owner:UNIVERSITY OF MISSOURI
Spinel catalysts for water and hydrocarbon oxidation
ActiveUS8932977B2Electrolytic capacitorsElectrical-based machining electrodesPhotoelectrochemical cellSpinel
A catalyst for the electrolysis of water molecules and hydrocarbons, the catalyst including catalytic groups comprising A1-xB2-yB′yO4 spinels having a cubical M4O4 core, wherein A is Li or Na, B and B′ are independently any transition metal or main group metal, M is B, B′, or both, x is a number from 0 to 1, and y is a number from 0 to 2. In photo-electrolytic applications, a plurality of catalytic groups are supported on a conductive support substrate capable of incorporating water molecules. At least some of the catalytic groups, supported by the support substrate, are able to catalytically interact with water molecules incorporated into the support substrate. The catalyst can also be used as part of a photo-electrochemical cell for the generation of electrical energy.
Owner:RUTGERS THE STATE UNIV
Biofuel composition and manufacturing process
InactiveUS8444845B2Minimize occurrenceReduces and prevents reactionElectrolysis componentsOrganic chemistryElectrolysisAlcohol
Owner:BUSCH RAINER
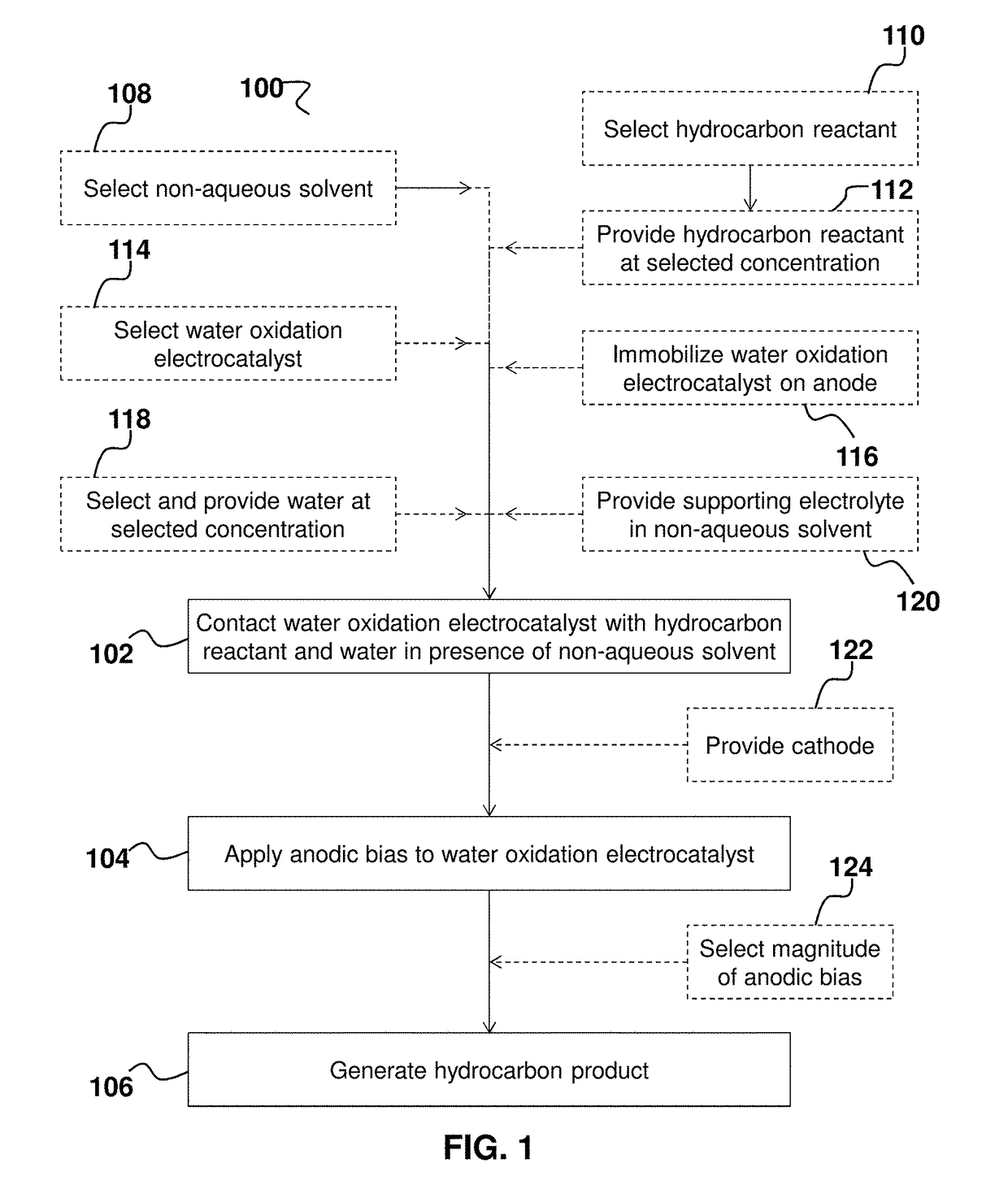
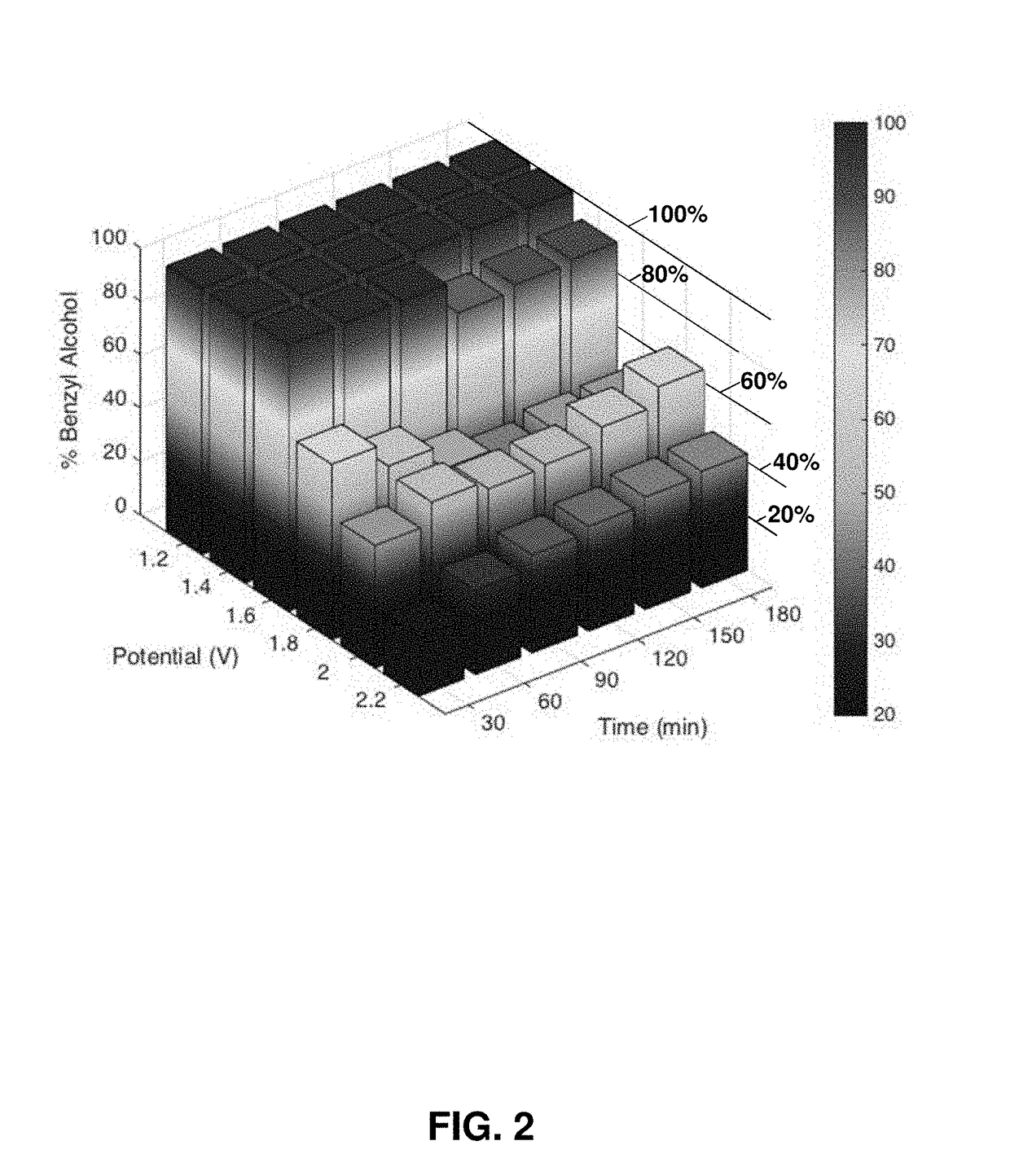
Hydrocarbon oxidation by water oxidation electrocatalysts in non-aqueous solvents
ActiveUS20180044804A1Prevent solubilizationAvoid miscibilityMultiple component coatingsElectrode with substrate and coatingSolventReagent
Provided herein are processes and systems for oxidation of a hydrocarbon reactant to generate an oxidized hydrocarbon product; said process comprising: contacting a water oxidation electrocatalyst with said hydrocarbon reactant and water in the presence of a non-aqueous solvent; wherein an anodic bias is applied to said water oxidation electrocatalyst, thereby generating said oxidized hydrocarbon product; and wherein said water oxidation electrocatalyst comprises one or more transition metals other than Ru. Optionally, said water is provided in said non-aqueous solvent at a concentration less than or equal to 0.5 vol. %. Optionally, the magnitude of said anodic bias is selected to generate said oxidized hydrocarbon product characterized by selected product distribution.
Owner:CALIFORNIA INST OF TECH
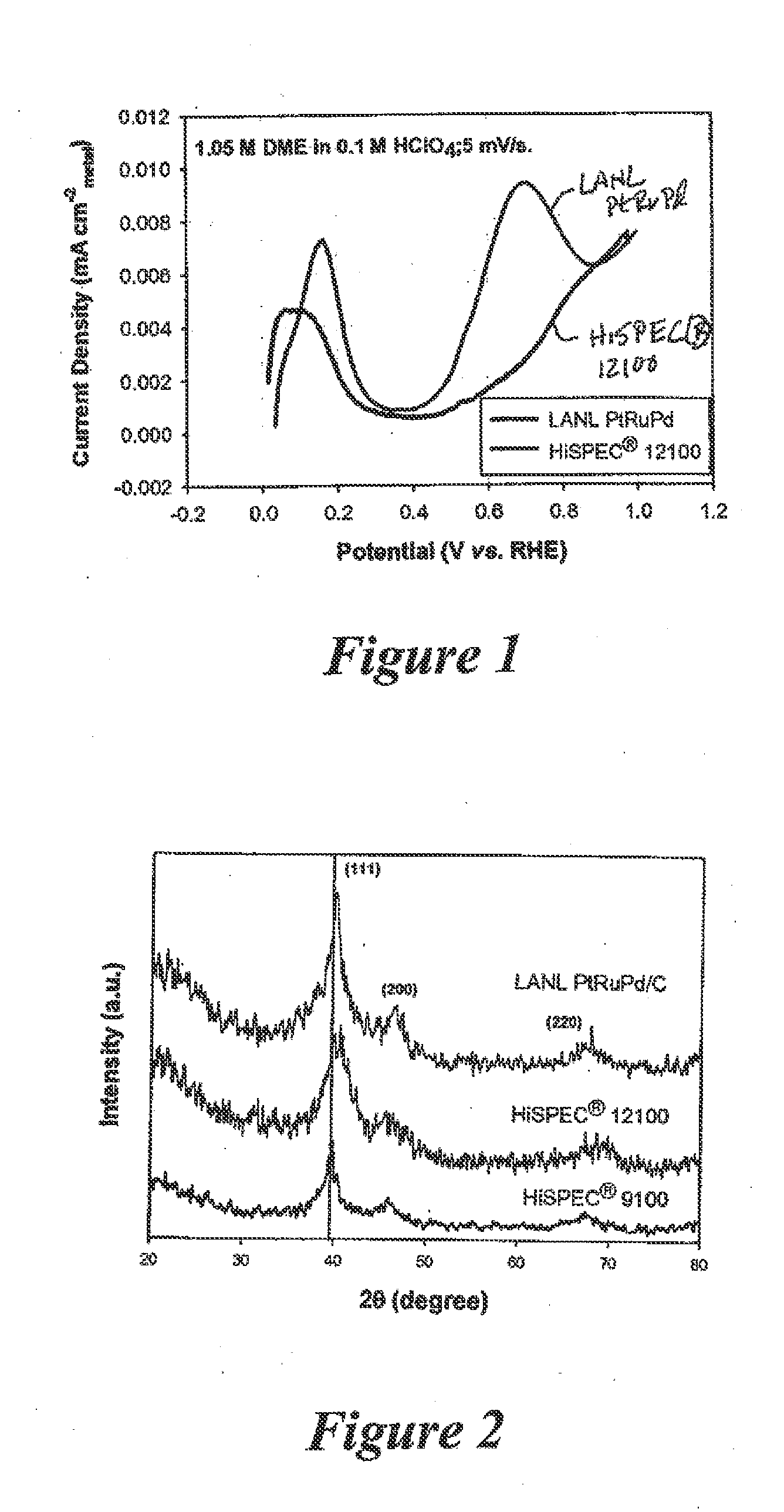
Catalytic Oxidation of Dimethyl Ether
A composition for oxidizing dimethyl ether includes an alloy supported on carbon, the alloy being of platinum, ruthenium, and palladium. A process for oxidizing dimethyl ether involves exposing dimethyl ether to a carbon-supported alloy of platinum, ruthenium, and palladium under conditions sufficient to electrochemically oxidize the dimethyl ether.
Owner:TRIAD NAT SECURITY LLC
Processes for producing oxide with higher oxidation than alcohol
InactiveUS7183421B2Efficiently oxidizedImprove efficiencyElectrolysis componentsOrganic compound preparationAlcoholSilica gel
The present invention provides a process for producing an oxide from an alcohol compound, the process comprising the steps of causing silica gel to carry the alcohol compound thereon and an oxidative catalyst thereon, and oxidizing the alcohol compound in the presence of an oxidizing agent, giving an oxide higher in oxidizing degree than the alcohol compound, and also provides a process for producing an oxide from an alcohol compound, the process comprising the steps of causing silica gel to carry the alcohol compound, and subjecting the alcohol compound to an electrolytic oxidation, giving an oxide higher in oxidizing degree than the alcohol compound.
Owner:OTSUKA CHEM CO LTD
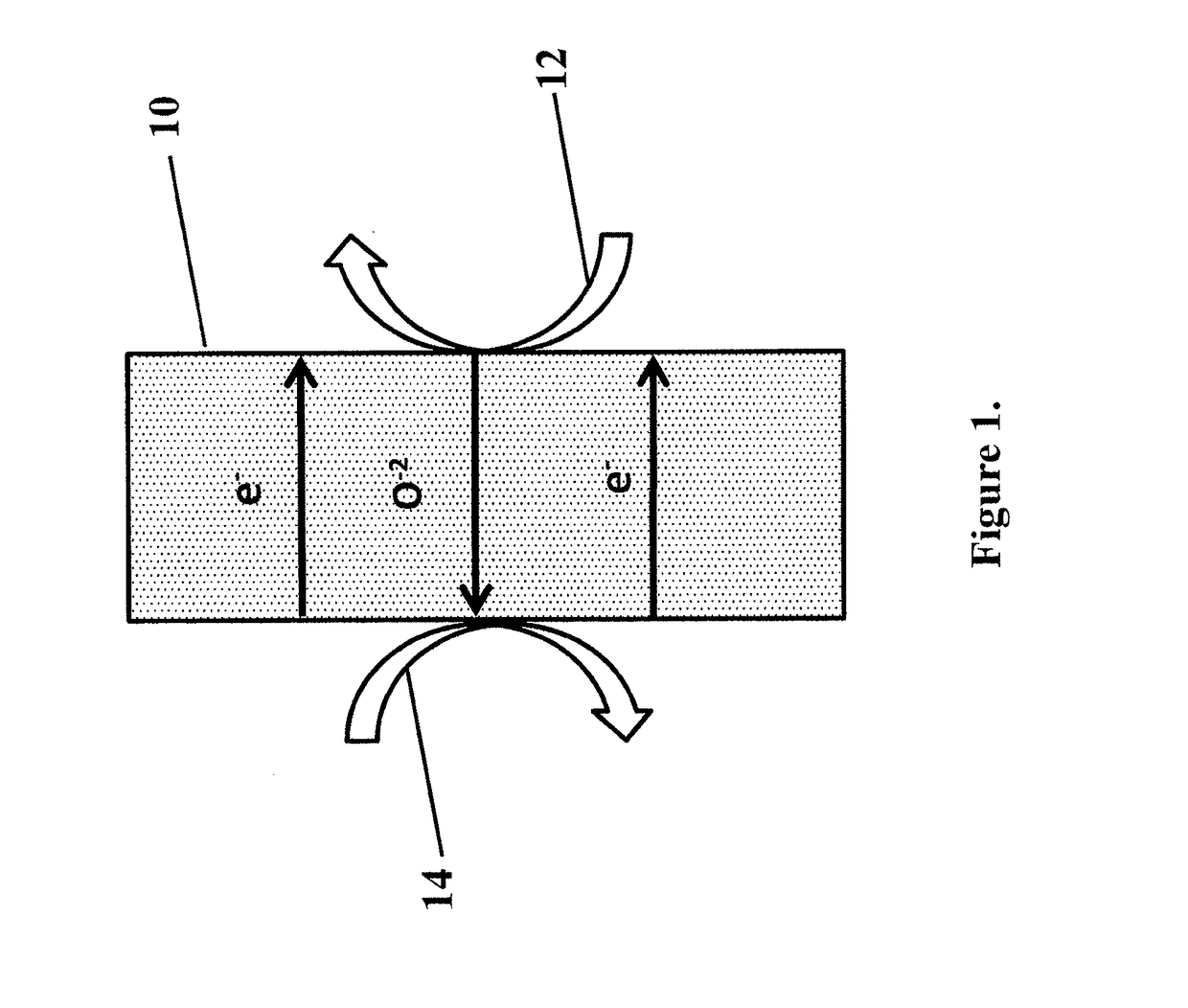
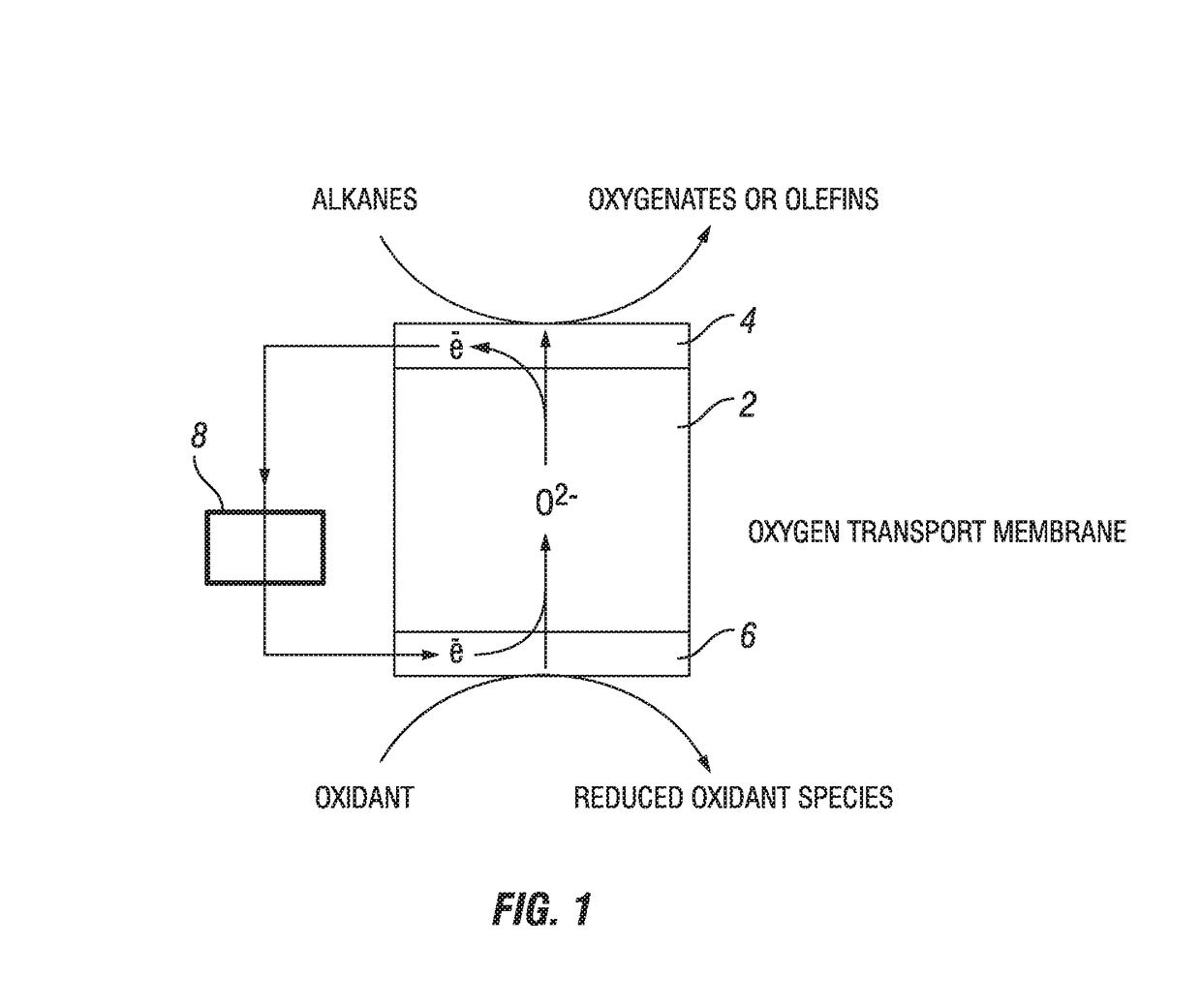
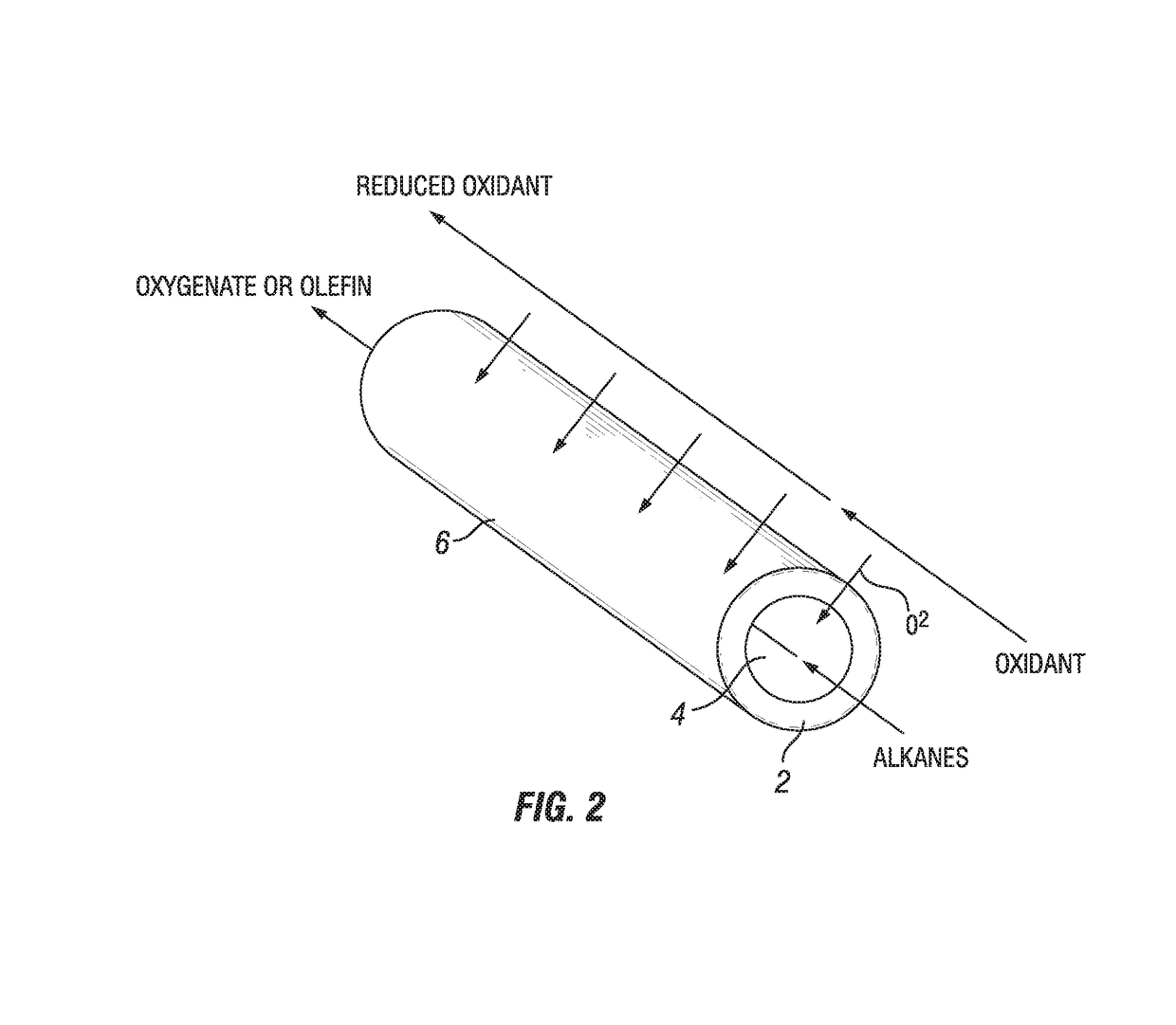
Process for oxidation reactions
The current embodiment describes a process of flowing an oxidant species over the reducing side of an oxygen transport membrane. O2− anions are then continuously transported from the reducing side through the oxygen transport membrane to the oxidizing side where an organic compound is converted to a partially oxidized organic compound on the oxidizing side.
Owner:PHILLIPS 66 CO
Selenium-based monomers and conjugated polymers, methods of making, and use thereof
Owner:UNIV OF CONNECTICUT
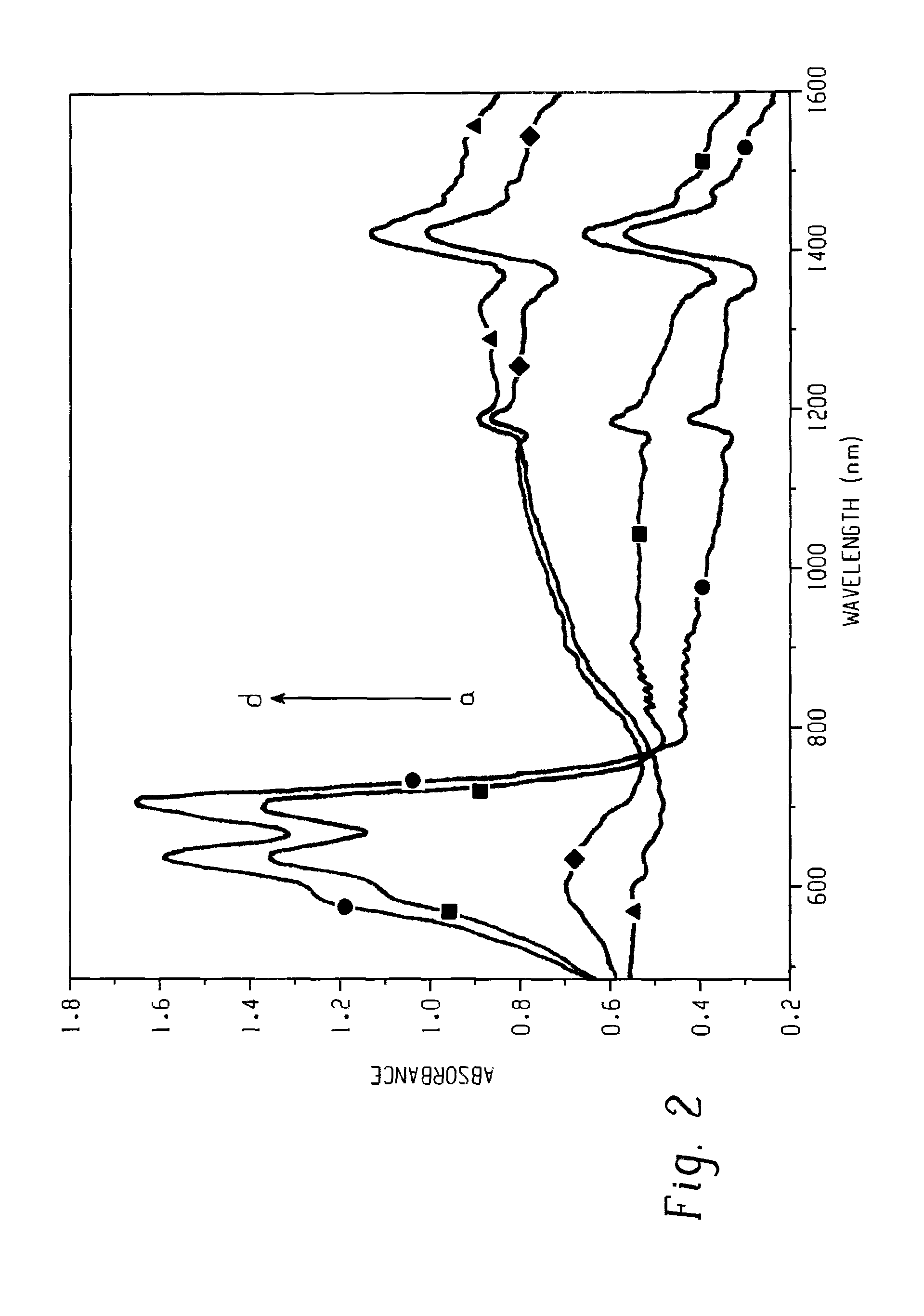
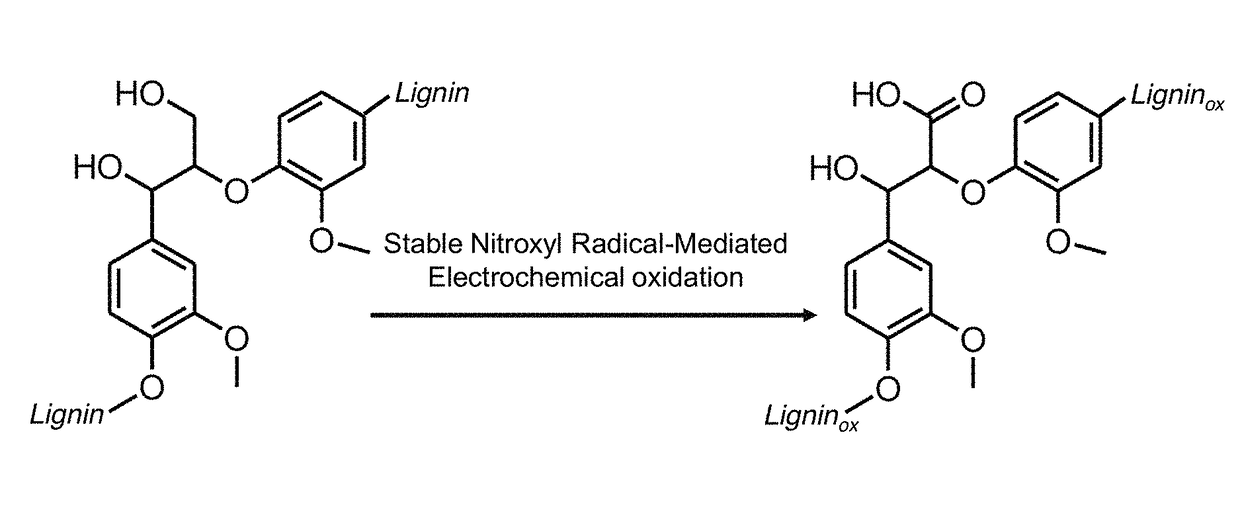
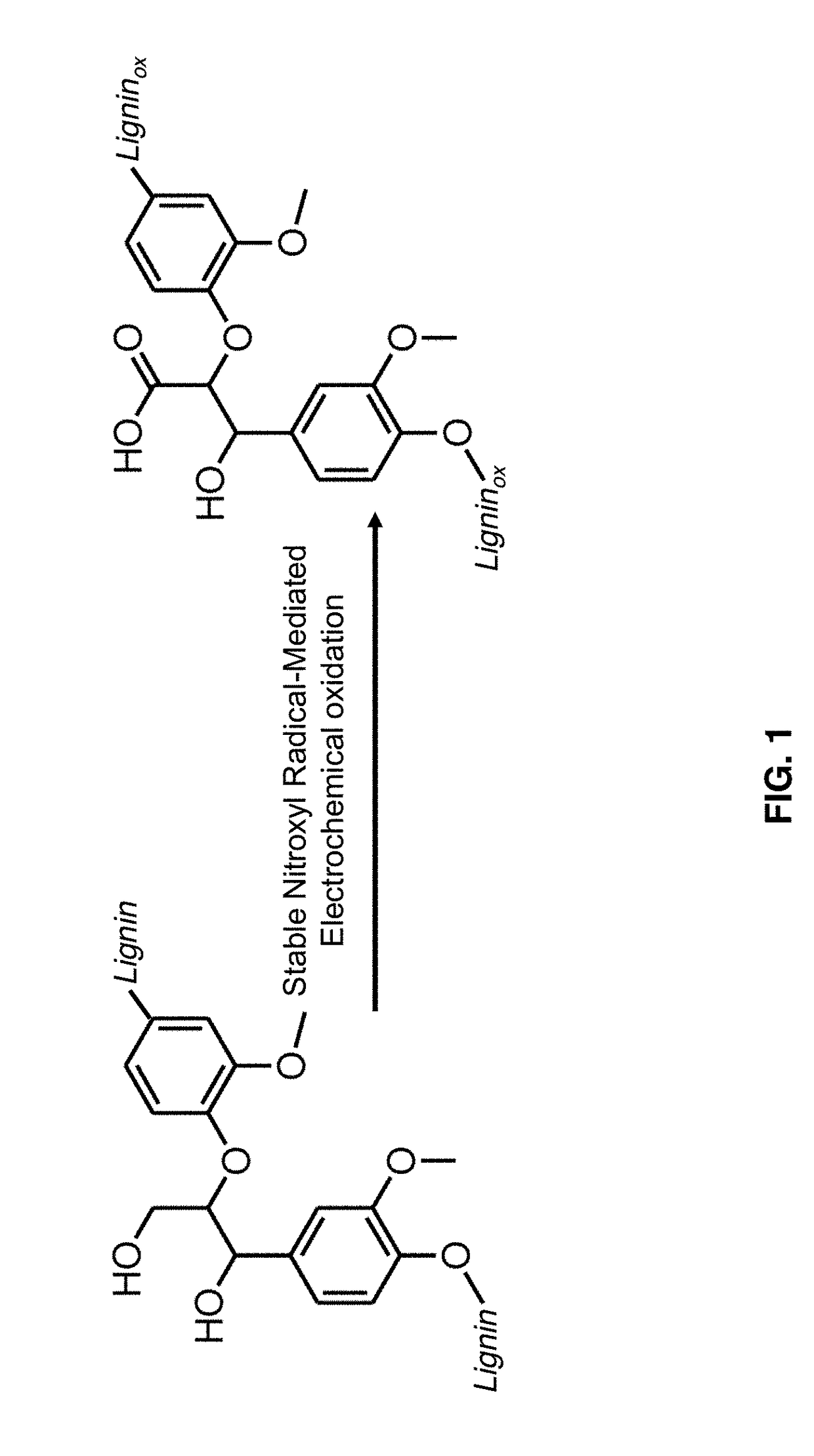
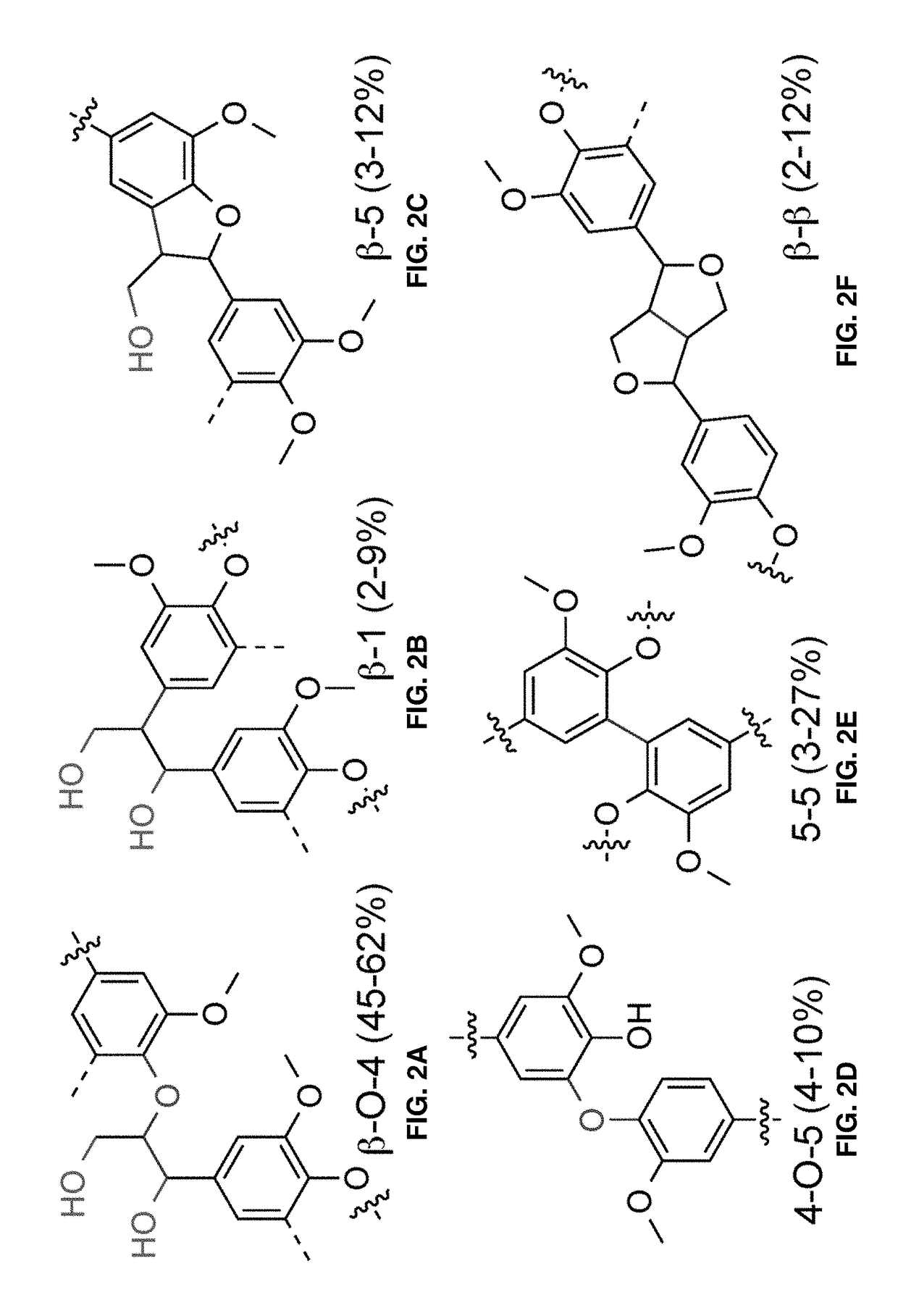
Nitroxyl-mediated oxidation of lignin and polycarboxylated products
ActiveUS9903028B2Increase productionElectrolysis componentsElectrolytic organic oxidationPhenylpropanoidProtonation
Owner:WISCONSIN ALUMNI RES FOUND
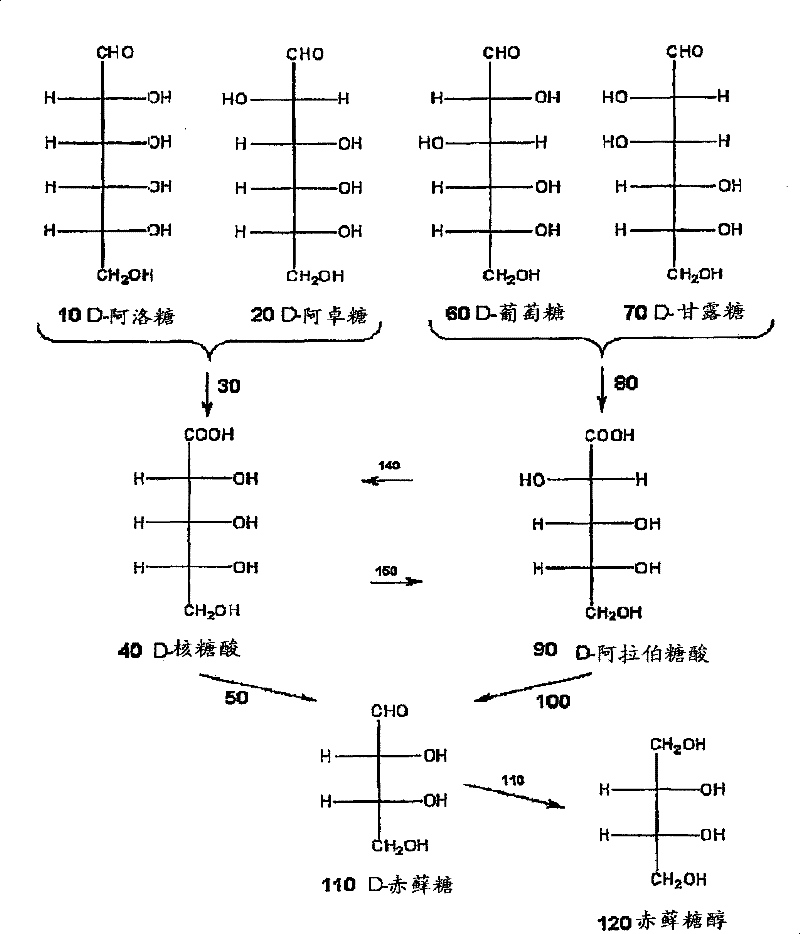
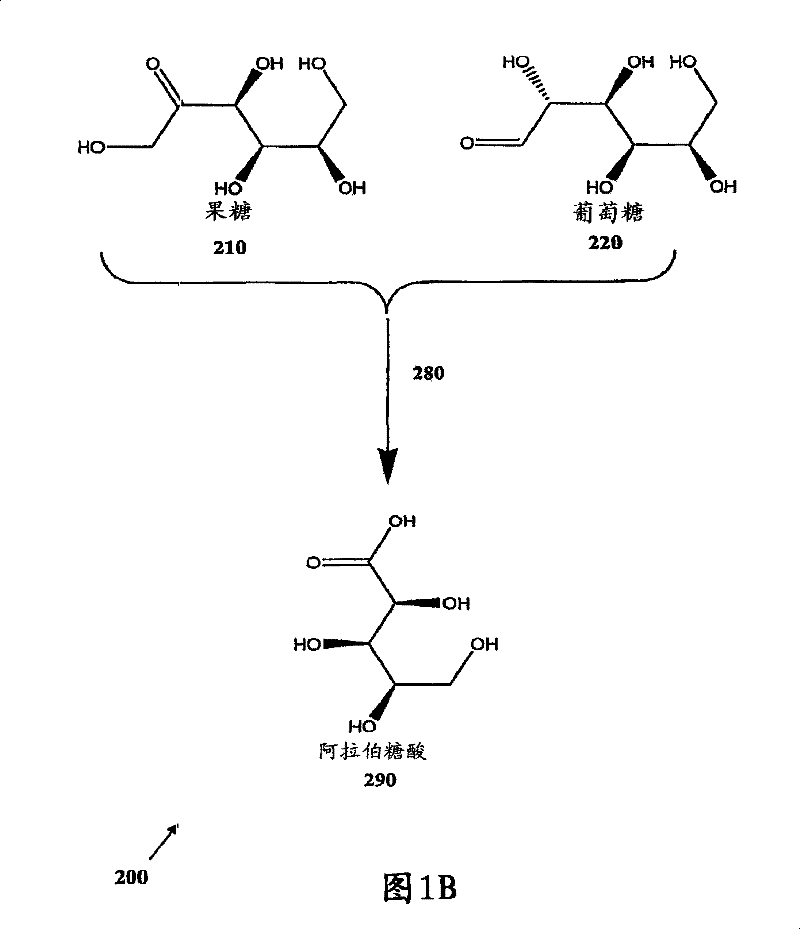
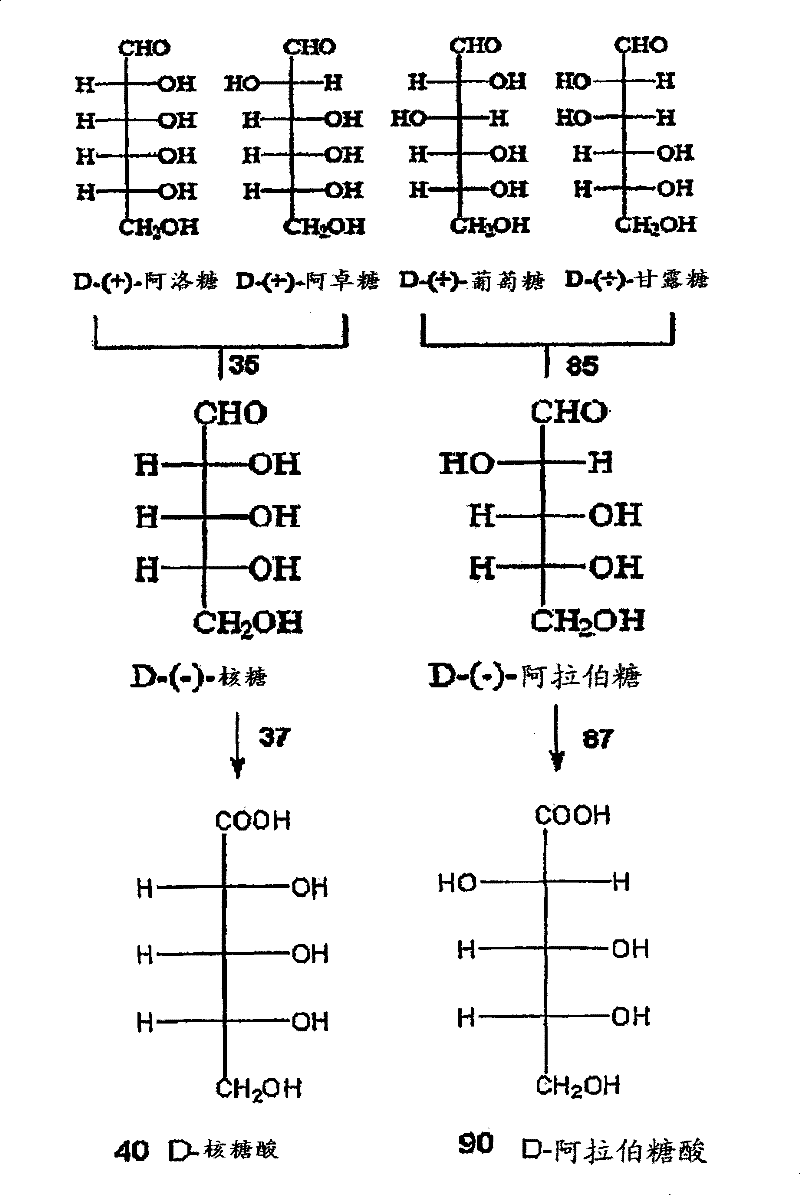
Methods for the electrolytic production of erythrose or erythritol
Owner:DFI USA LLC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com