Patents
Literature
266results about "Electrogenerative processes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
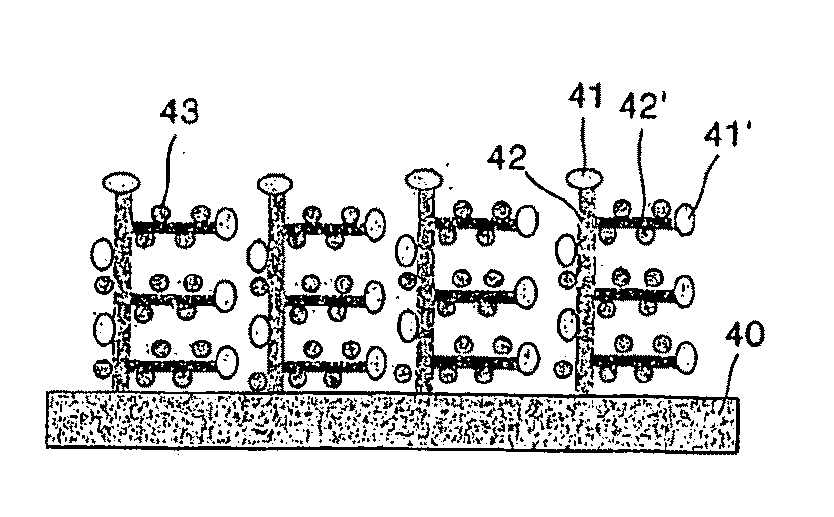
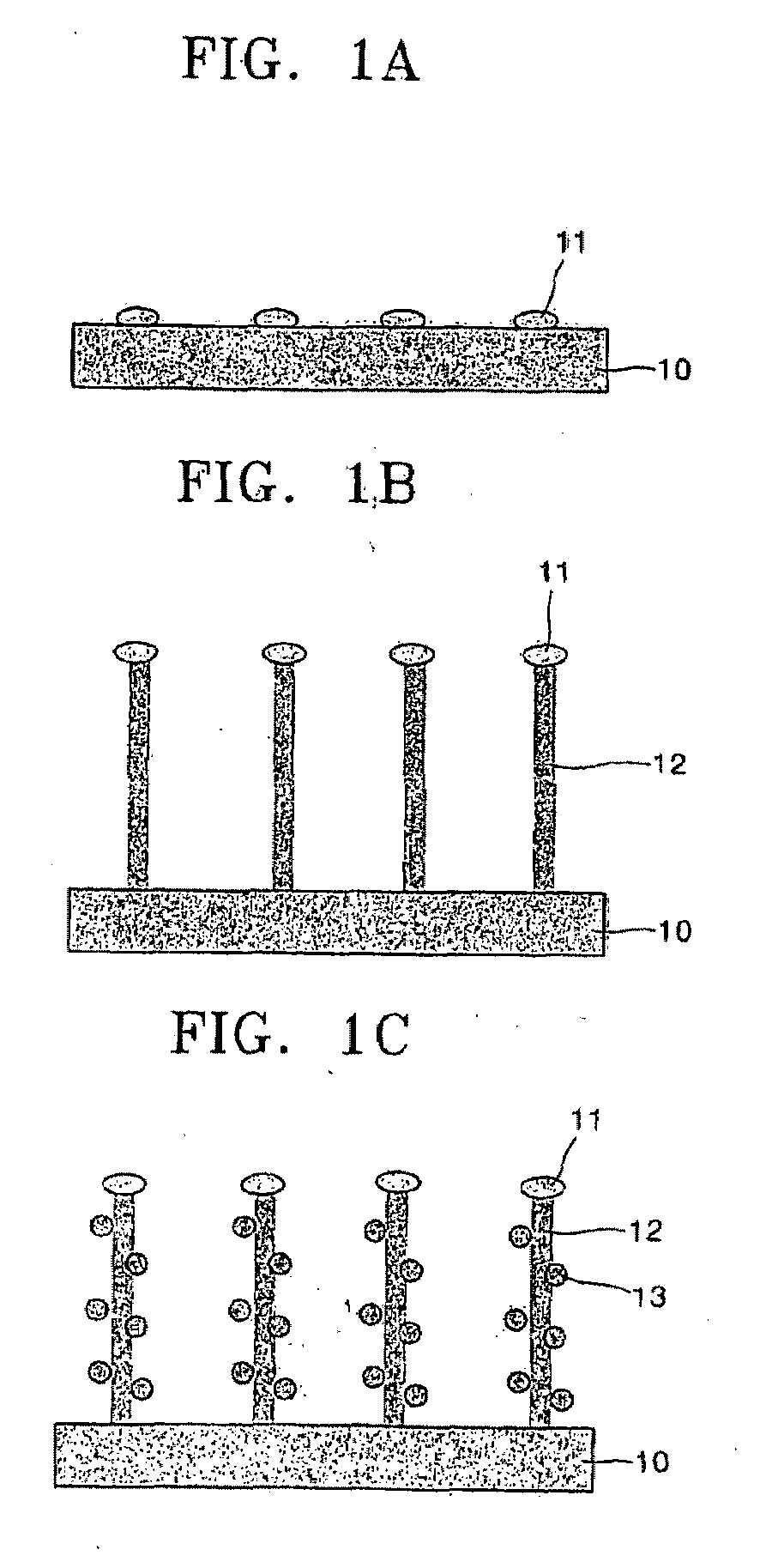
Carbon nanotube for fuel cell, nanocomposite comprising the same, method for making the same, and fuel cell using the same
InactiveUS20090075157A1High gas-reaction efficiencyImprove efficiencyMaterial nanotechnologyLamination ancillary operationsFuel cellsNanoparticle
Provided are aligned carbon nanotubes for a fuel cell having a large surface area, a nanocomposite that includes the aligned carbon nanotubes loaded with highly dispersed nanoparticles of a metallic catalyst, methods of producing the carbon nanotubes and the nanocomposite, and a fuel cell including the nanocomposite. In the nanocomposite, nanoparticles of the metallic catalyst are uniformly distributed on external walls of the nanotubes. A fuel cell including the nanocomposite exhibits better performance.
Owner:RGT UNIV OF CALIFORNIA
Method for agile workpiece temperature control in a plasma reactor using a thermal model
ActiveUS20070091537A1Reduce the differenceElectric discharge tubesDecorative surface effectsTemperature controlProcess engineering
A method of processing a workpiece in a plasma reactor having an electrostatic chuck for holding a workpiece in a chamber of the reactor includes providing a thermally conductive gas under pressure between a backside of the workpiece and a top surface of the electrostatic chuck, controlling the temperature of the electrostatic chuck, defining a desired workpiece temperature, measuring a current workpiece temperature or temperature related to the workpiece temperature and inputting the measured temperature to a thermal model representative of the electrostatic chuck. The method further includes determining from the thermal model a change in the pressure of the thermally conductive gas that would at least reduce the difference between the measured temperature and the desired temperature, and changing the pressure of the thermally conductive gas in accordance with the change determined from the thermal model.
Owner:ADVANCED THERMAL SCI
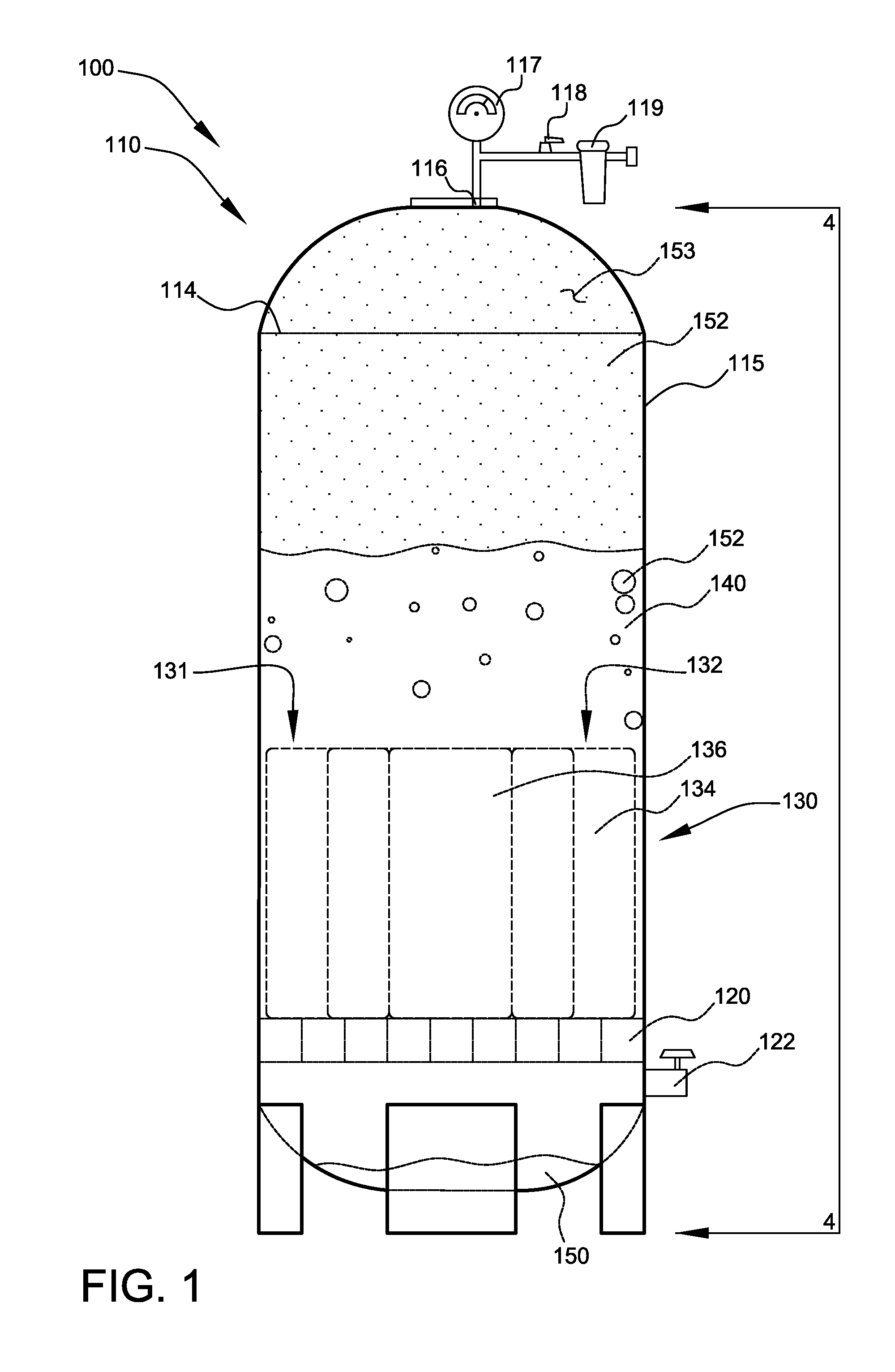
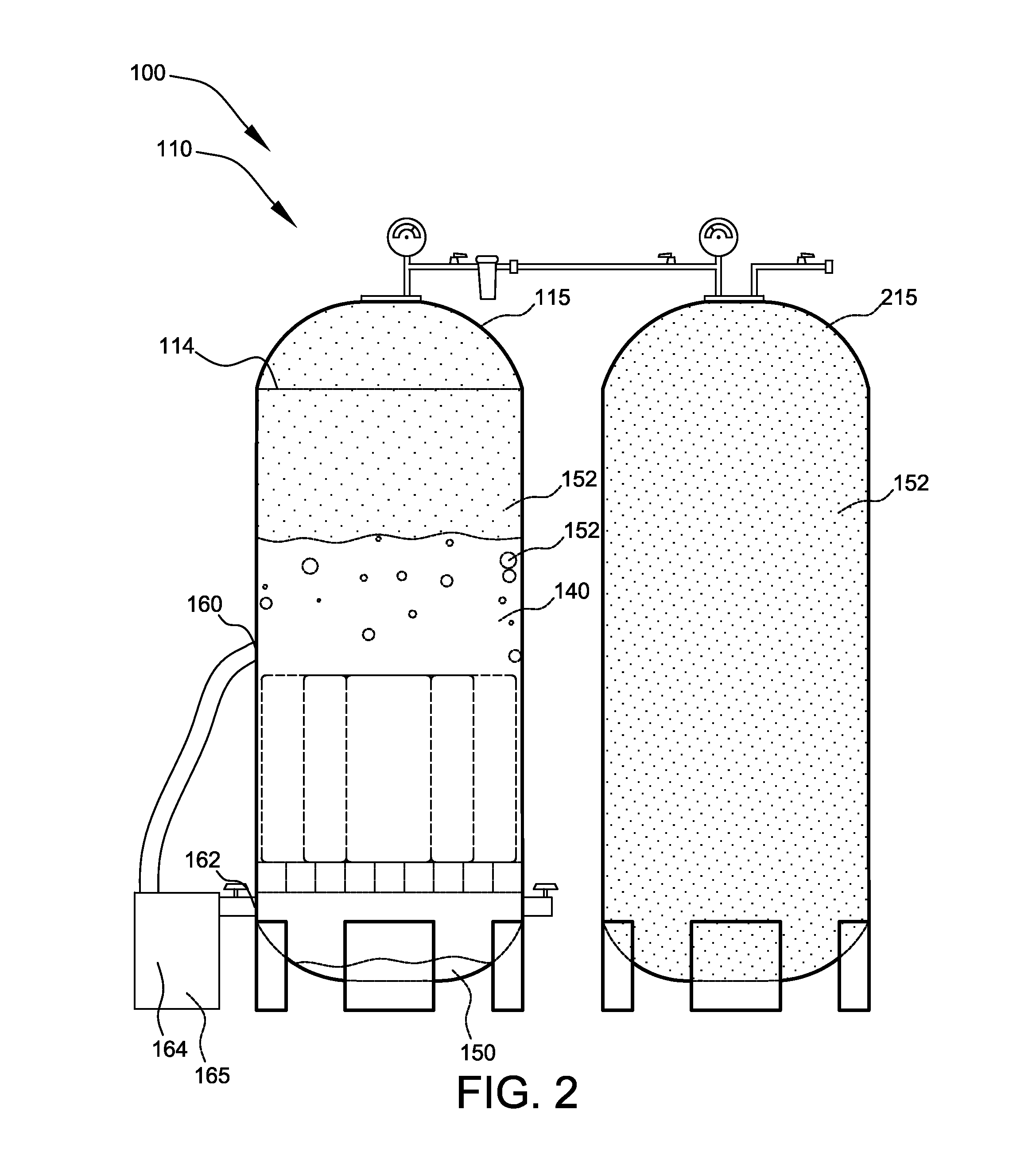
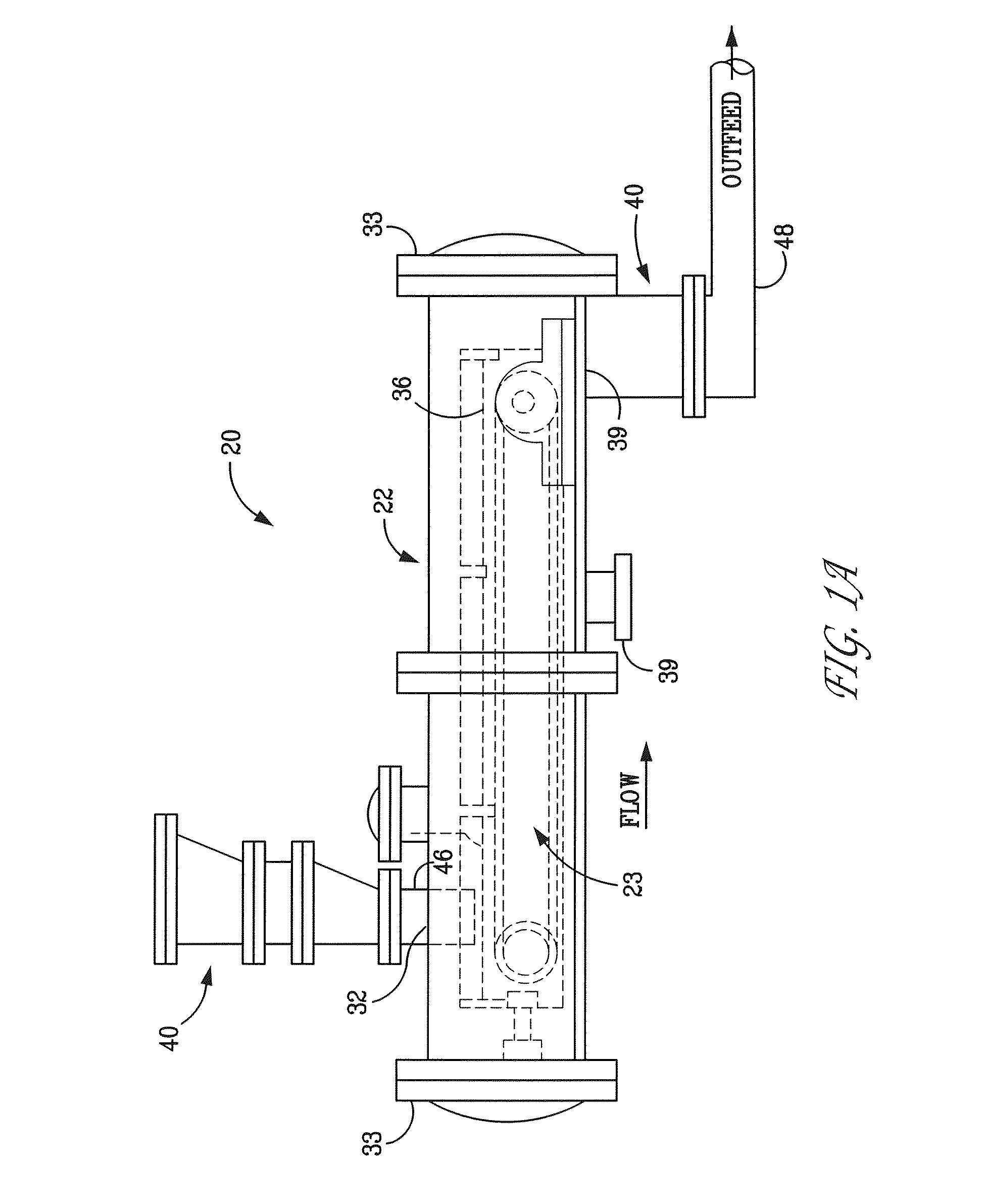
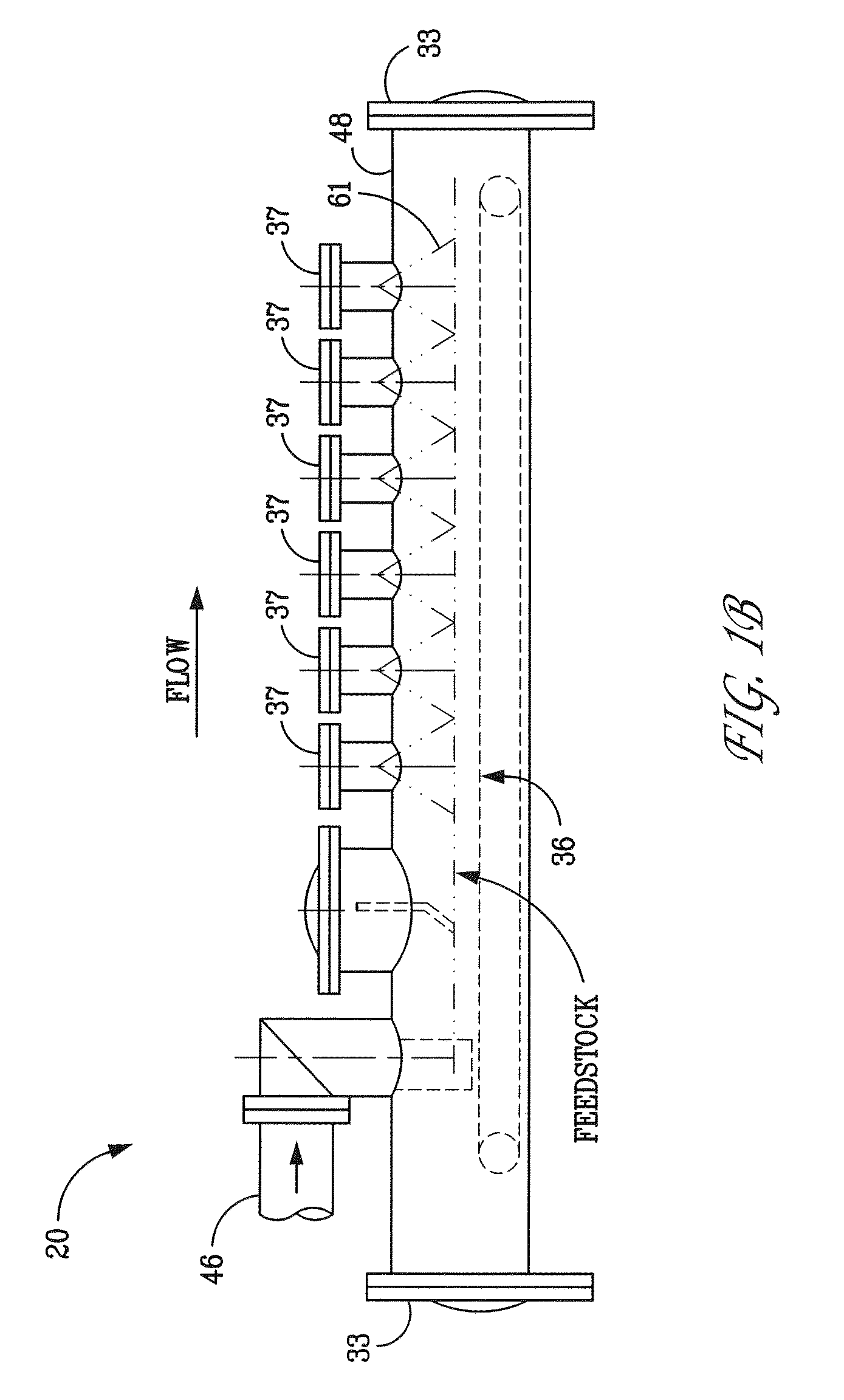
Method and apparatus for generating hydrogen
An electro-galvanic hydrogen generator system that has two or more anode materials; a cathode material; and an electrolyte. The electrolyte comprises a metal hydride, at least one stabilizing agent, and a solvent. Hydrogen gas is generated whenever an anode material and the cathode material are electrically connected, and the different anode materials can be used separately or in combination to control the quantity and rate of hydrogen generation. An insulating shield may be used to catch reaction debris from the anodes. A removable inert layer may cover the electrodes. The use of cell pressure may regulate the rate of hydrogen generation. The hydrogen generator may have a separate catalyst chamber, and the hydrogen generator may also function as a battery.
Owner:PETILLO PHILLIP J
Water repellent metal-organic frameworks, process for making and uses regarding same
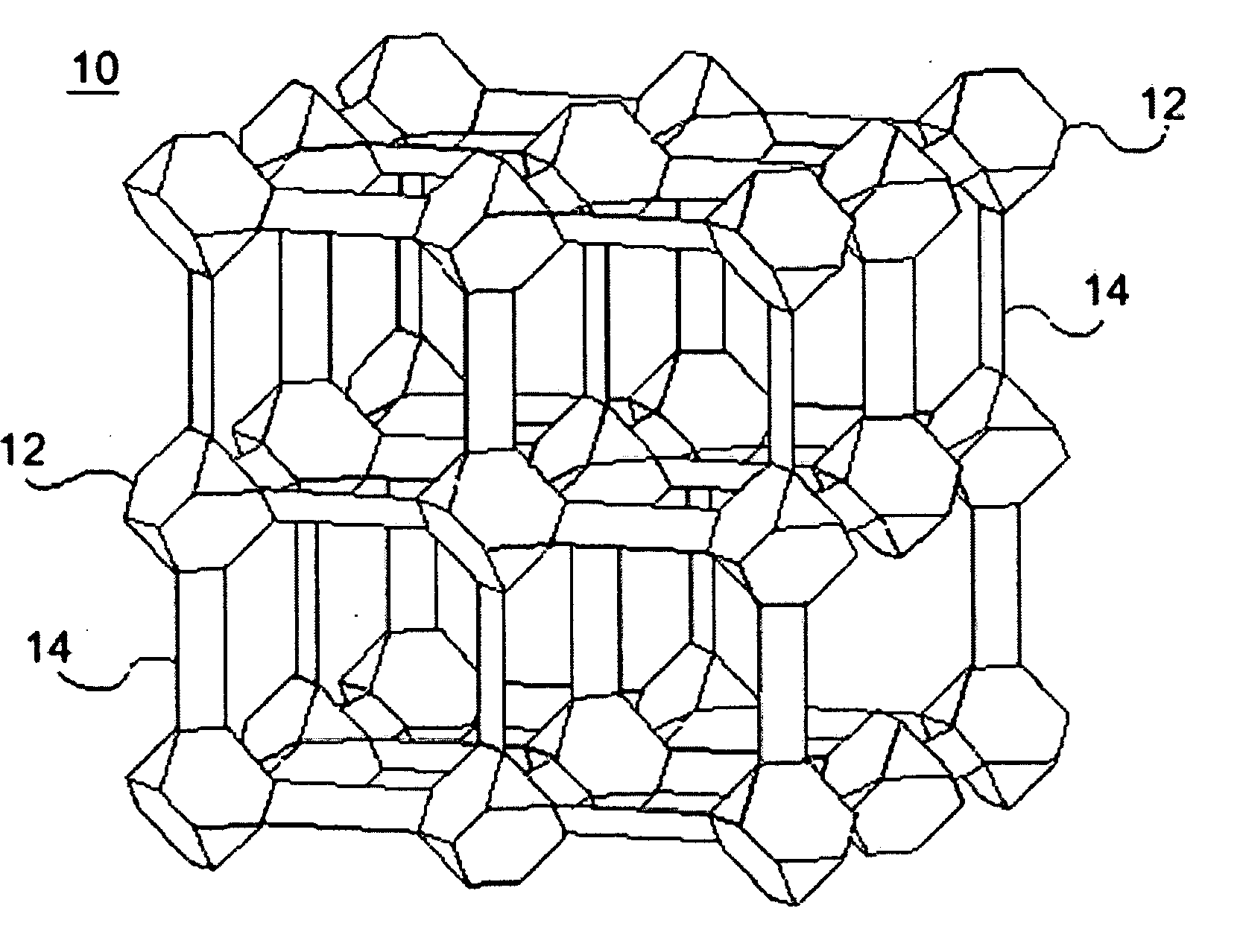
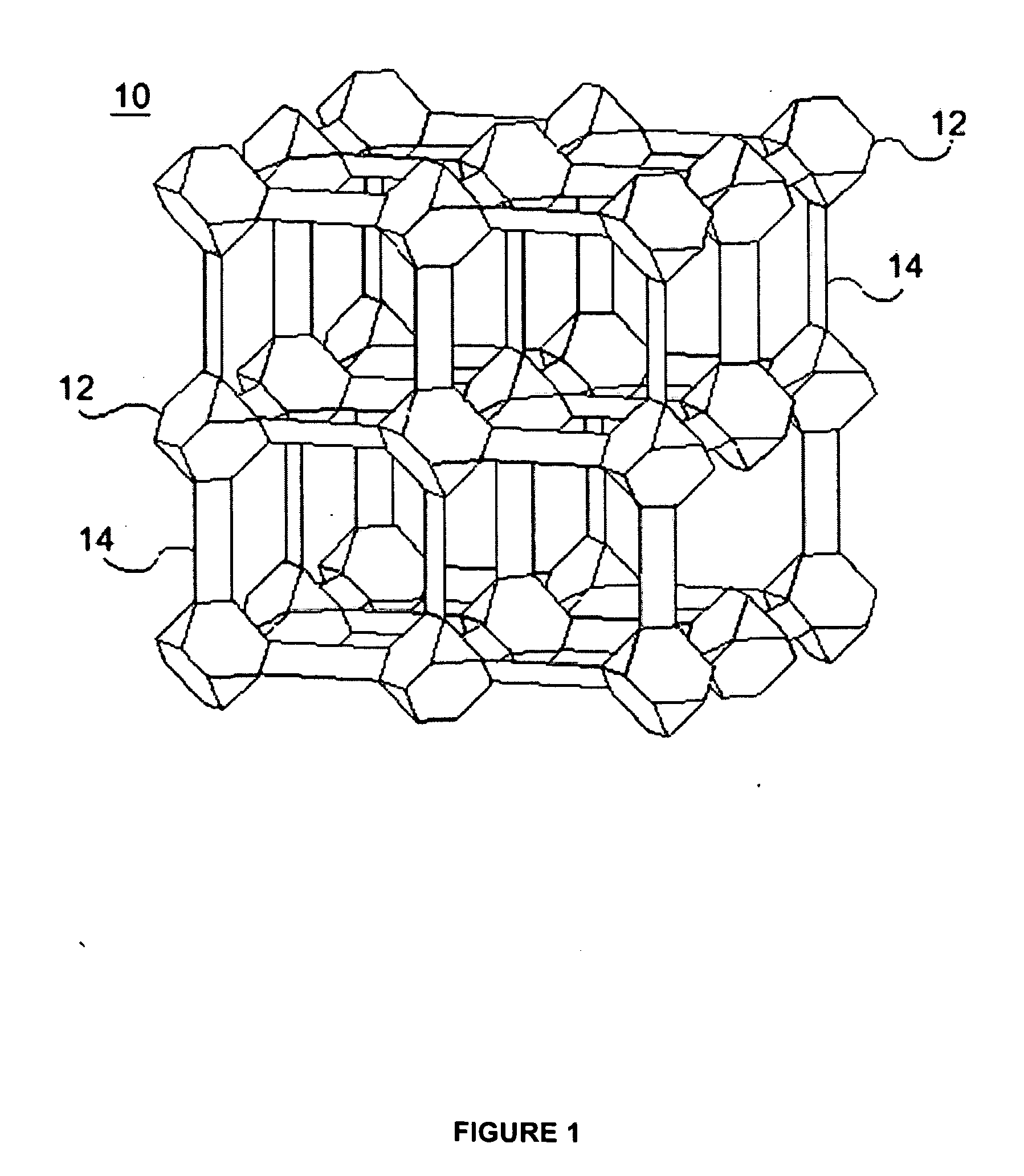
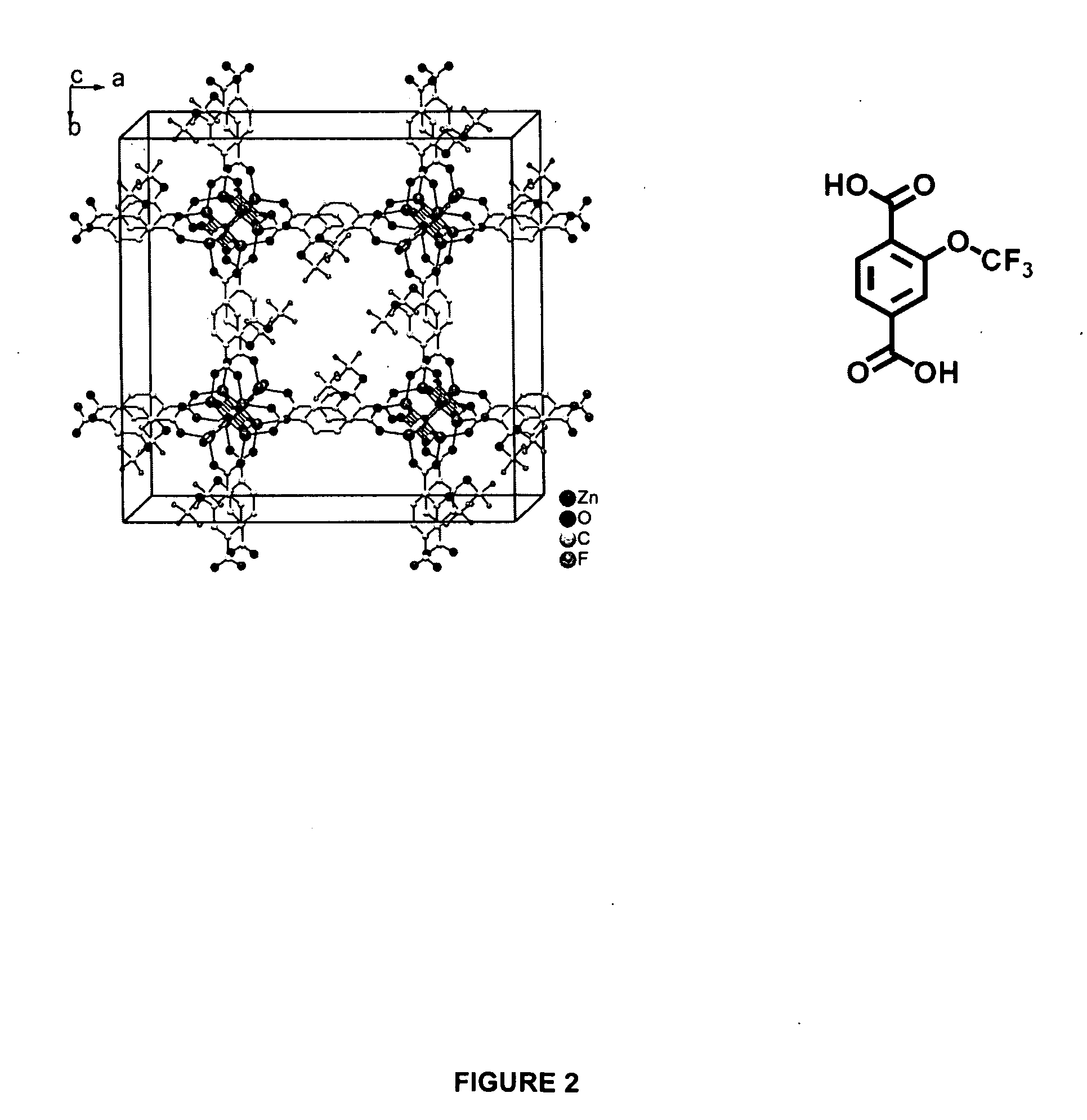
ActiveUS20100075123A1Improve stabilityImproved MOF stabilityGroup 8/9/10/18 element organic compoundsLayered productsWater vaporSorbent
Microwave assisted synthesis may be used to produce water-repellent metallic organic frameworks (MOFs) molecules. The water-repellent MOFs contain non-polar functional groups, such as a trifluoromethoxy group, which has a strong water repellent effect. The water-repellent MOF, when exposed to water vapor for one week does not result in a significant X-ray power pattern change. The water-repellent MOFs may be suitable as an adsorbent in many industrial applications, such as gas chromatography.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Integrated renewable energy system
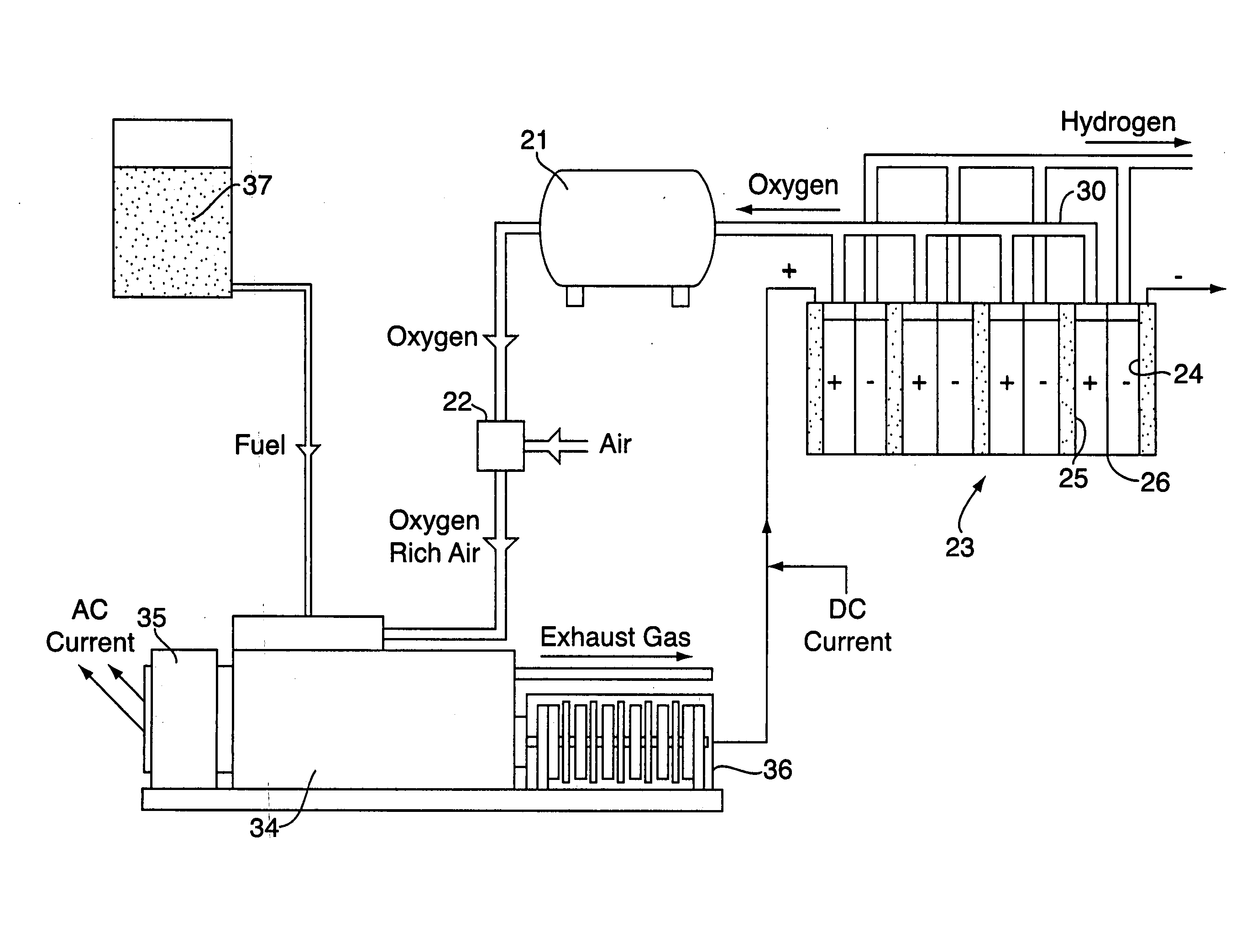
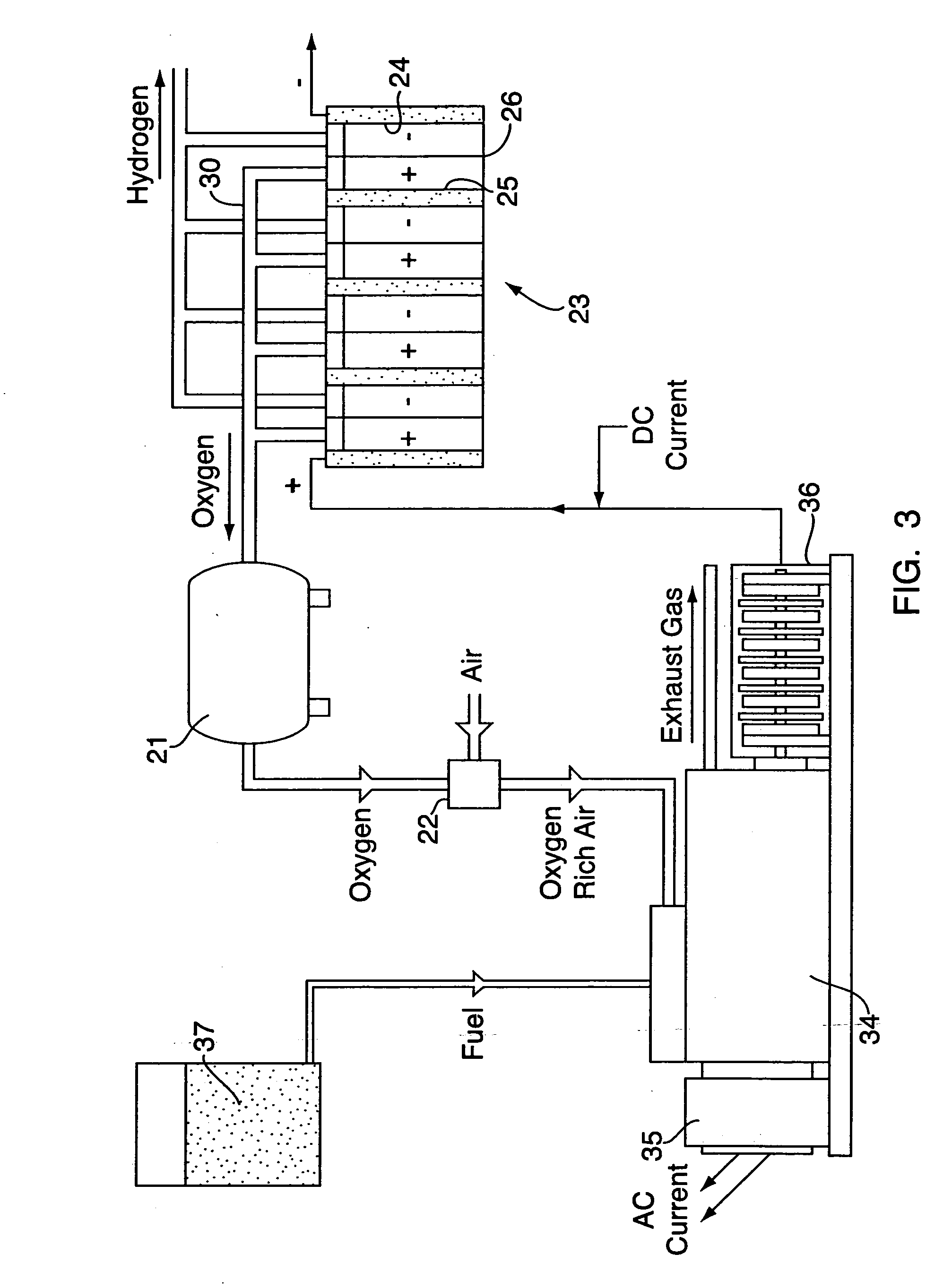
InactiveUS20070001462A1Reduce environmental pollutionFuel efficiencyElectrolysis componentsInternal combustion piston enginesElectrolysisOxygen
A system and method are disclosed comprising coupling a compression ignition engine (17) to an AC electrical generator (18) and / or a DC hompolar generator (19), wherein the homopolar generator (19) produces an electric current which is used to electrolyse water into hydrogen and oxygen. The hydrogen from the water electrolysis process may be used as a renewable fuel, either in the form of a gaseous fuel or a reactant in a fuel cell. The oxygen from the water electrolysis unit (23) may be used to produce an oxygen enriched combustion atmosphere in the engine (17). The oxygen may optionally be used as a reactant, along with the hydrogen, in a fuel cell.
Owner:H EMPOWER CORP
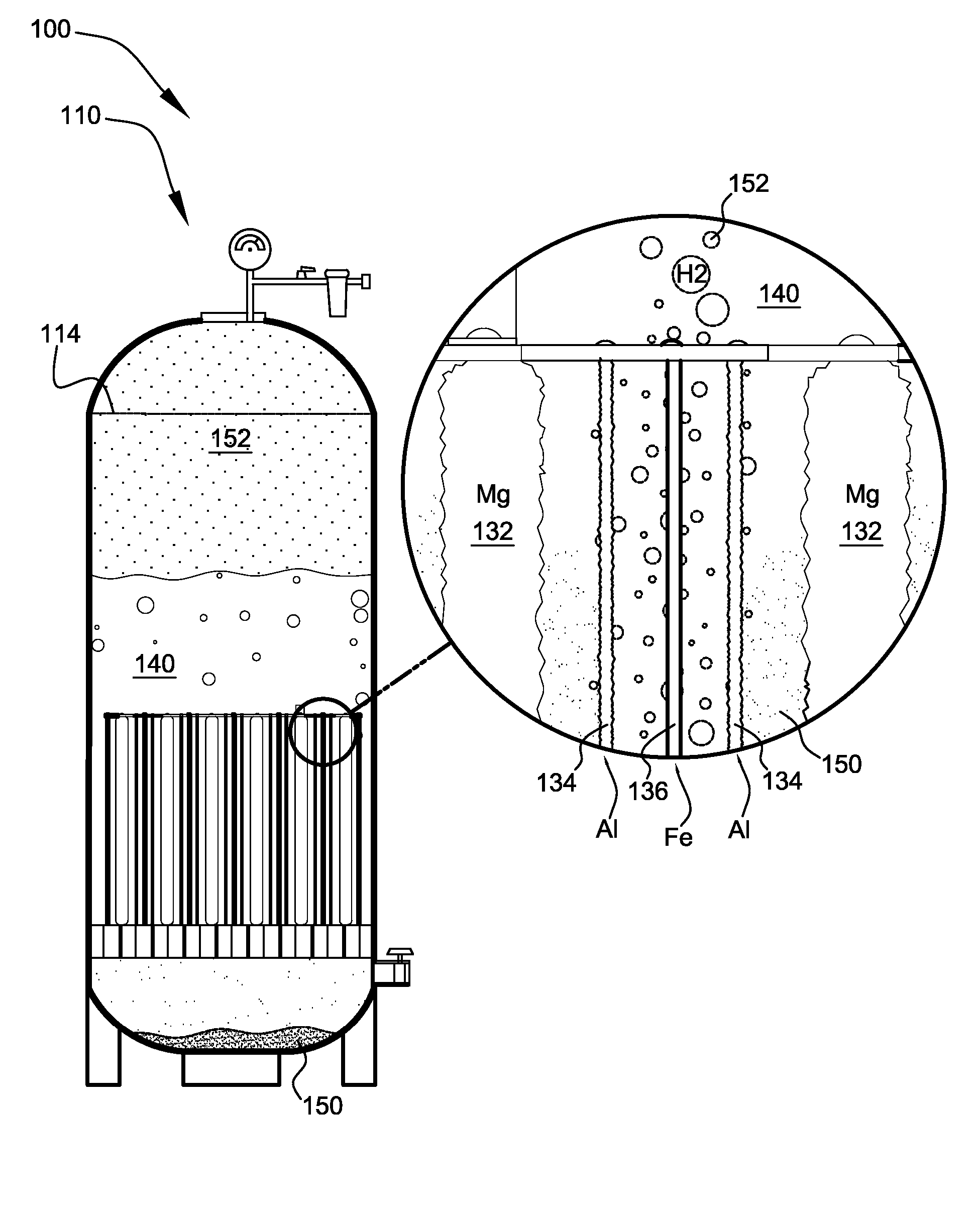
Hydrogen Energy Systems
InactiveUS20070039815A1Avoid overall overheatingEfficiently safely easily provideCellsAluminium compoundsPetroleumDiesel engine
A hydrogen energy system comprising galvanic hydrogen generators and hydrogen input manifolds for vehicle engines. The galvanic hydrogen generators generate hydrogen gas, magnesium hydroxide, and heat by the galvanic reaction of magnesium anodes with steel cathodes in salt water. Heat exchangers channel excess heat to a heat sink such as a thermocouple, Stirling engine, hot water system, etc. The hydrogen input manifold is bolted between the engine block and the air intake manifold of a gasoline or diesel engine. Hydrogen gas is injected into the hydrogen input manifold to provide supplementary fuel to the engine, lowering the amount of petroleum that is used.
Owner:BARTEL BRIAN G
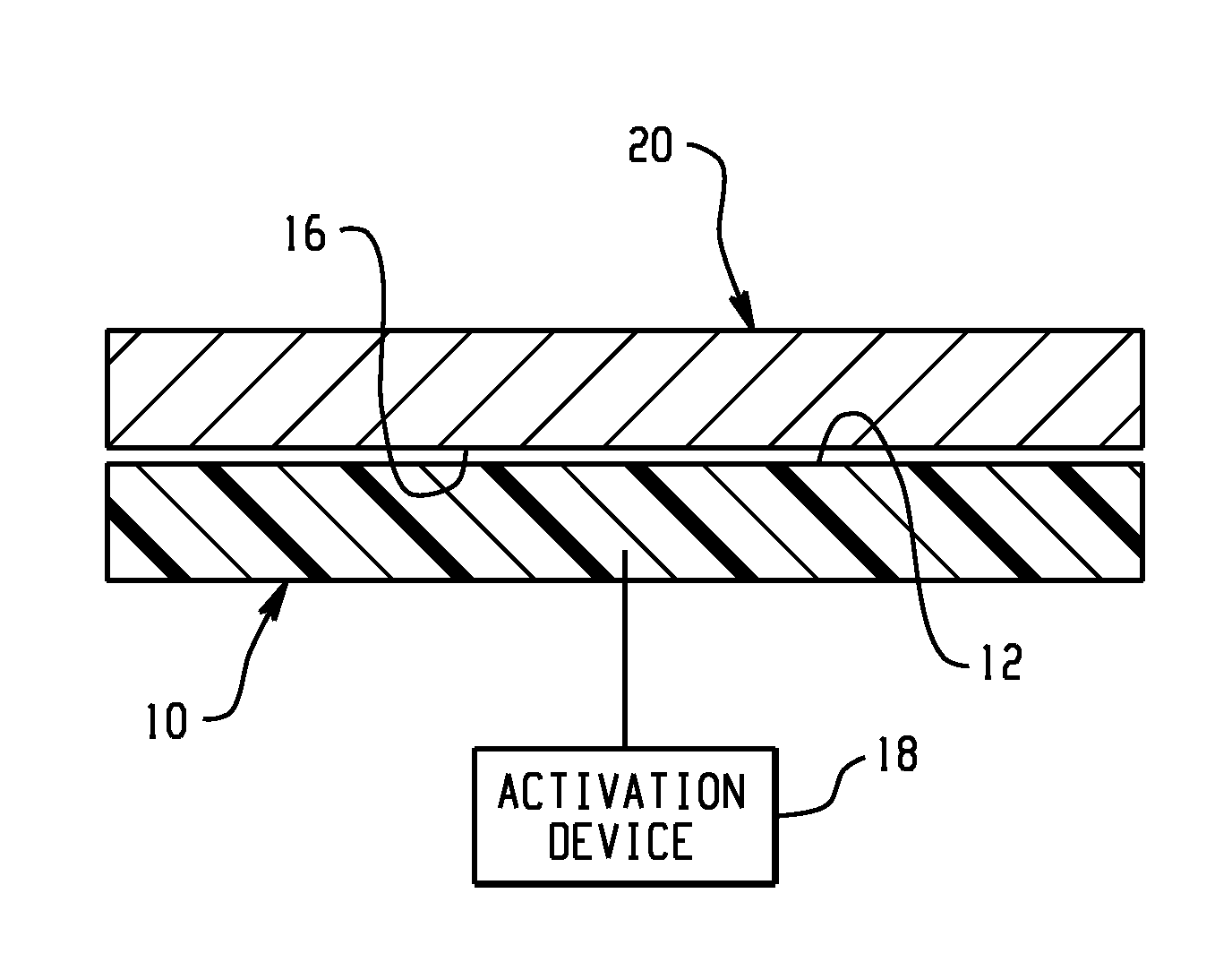
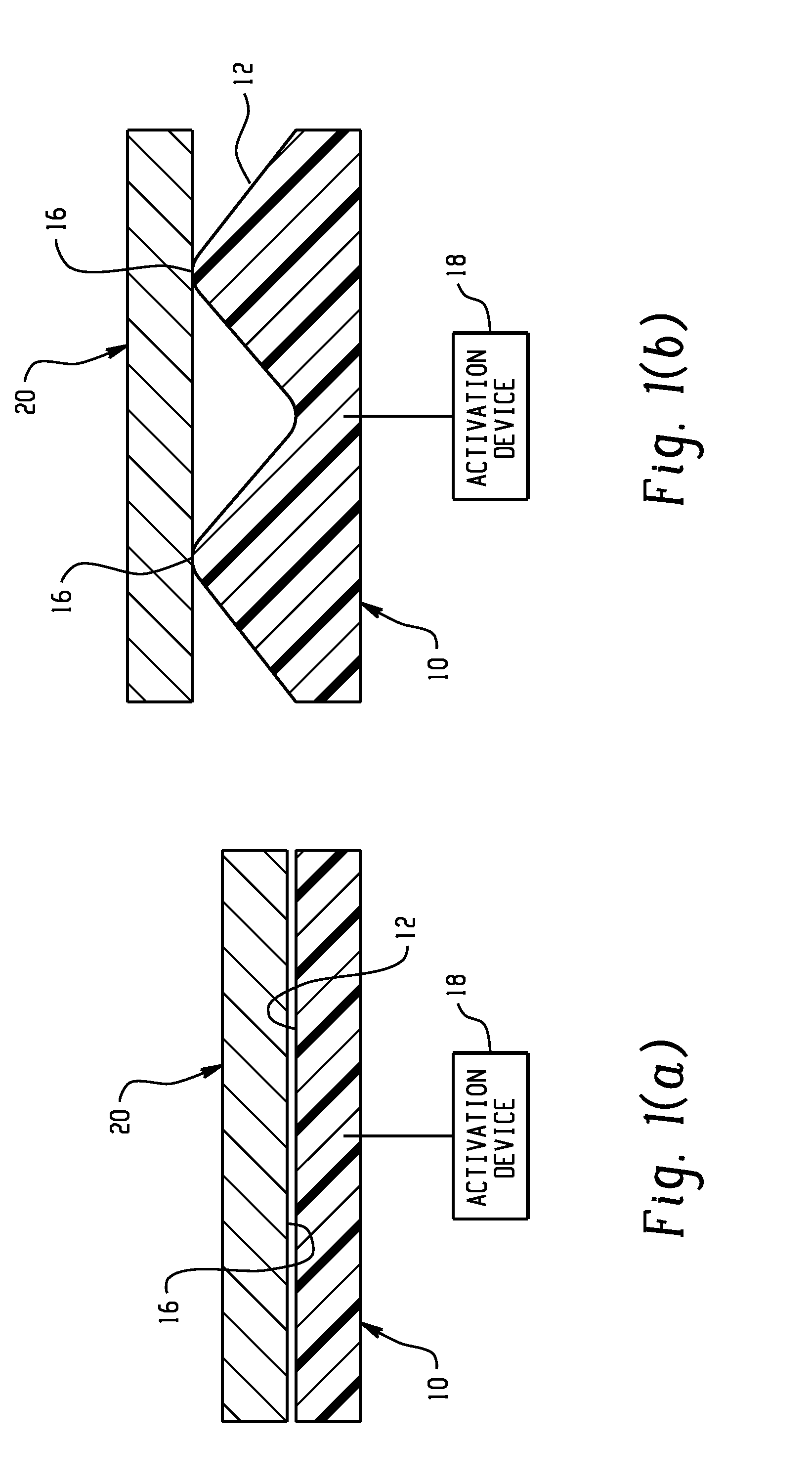
Active material based bodies for varying frictional force levels at the interface between two surfaces
A device for selectively controlling and varying a frictional force level at an interface between two bodies, includes a first contact body having at least one surface, a second contact body having at least one surface in physical communication with the first contact body, and an active material in operative communication with a selected one or both of the first contact body and the second contact body, wherein the active material is configured to undergo a change in a property upon receipt of an activation signal wherein the change in a property is effective to change the frictional force level at the interface between the at least one surface of the first contact body and the at least one surface of the second contact body.
Owner:GM GLOBAL TECH OPERATIONS LLC
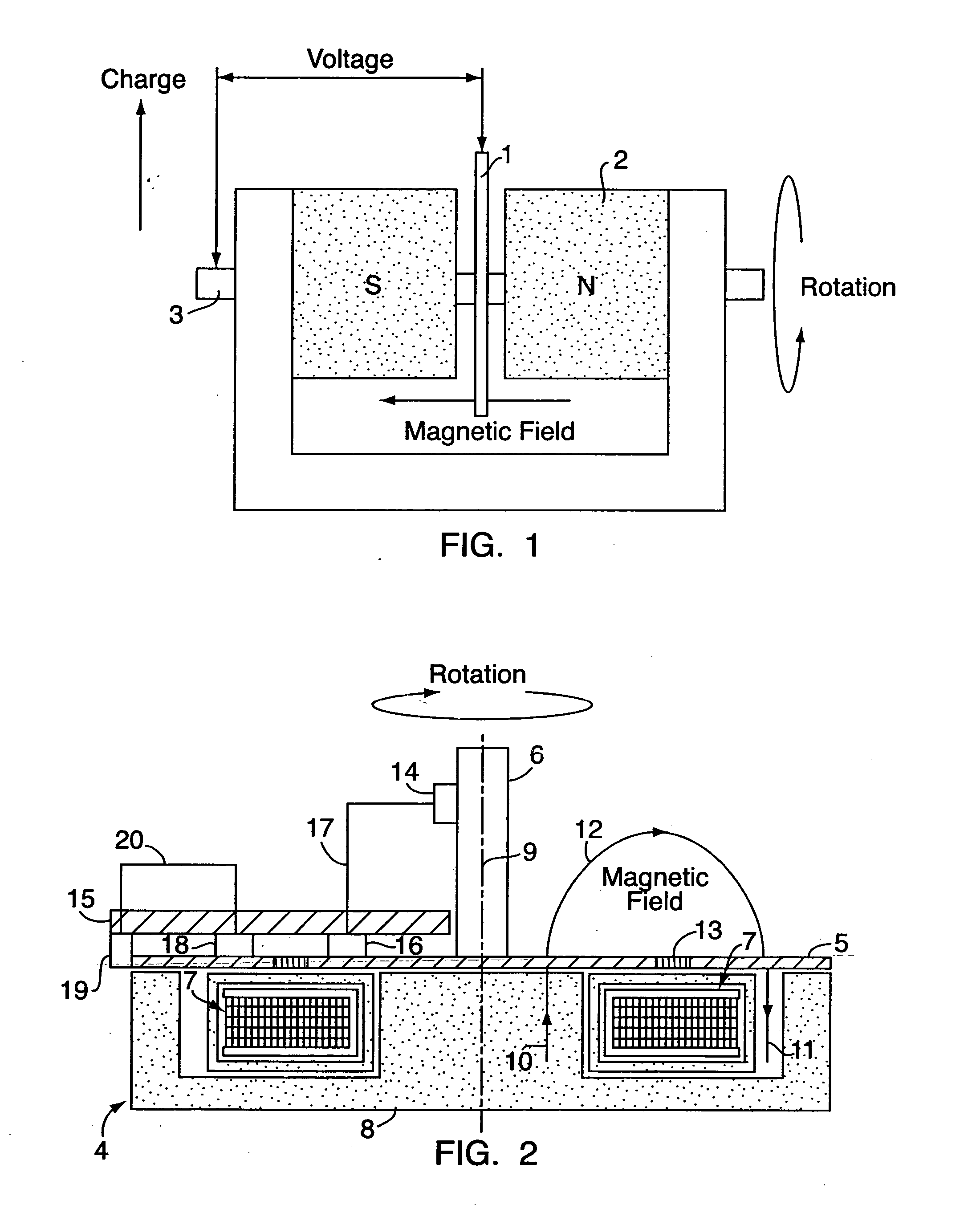
Liquid treatment device
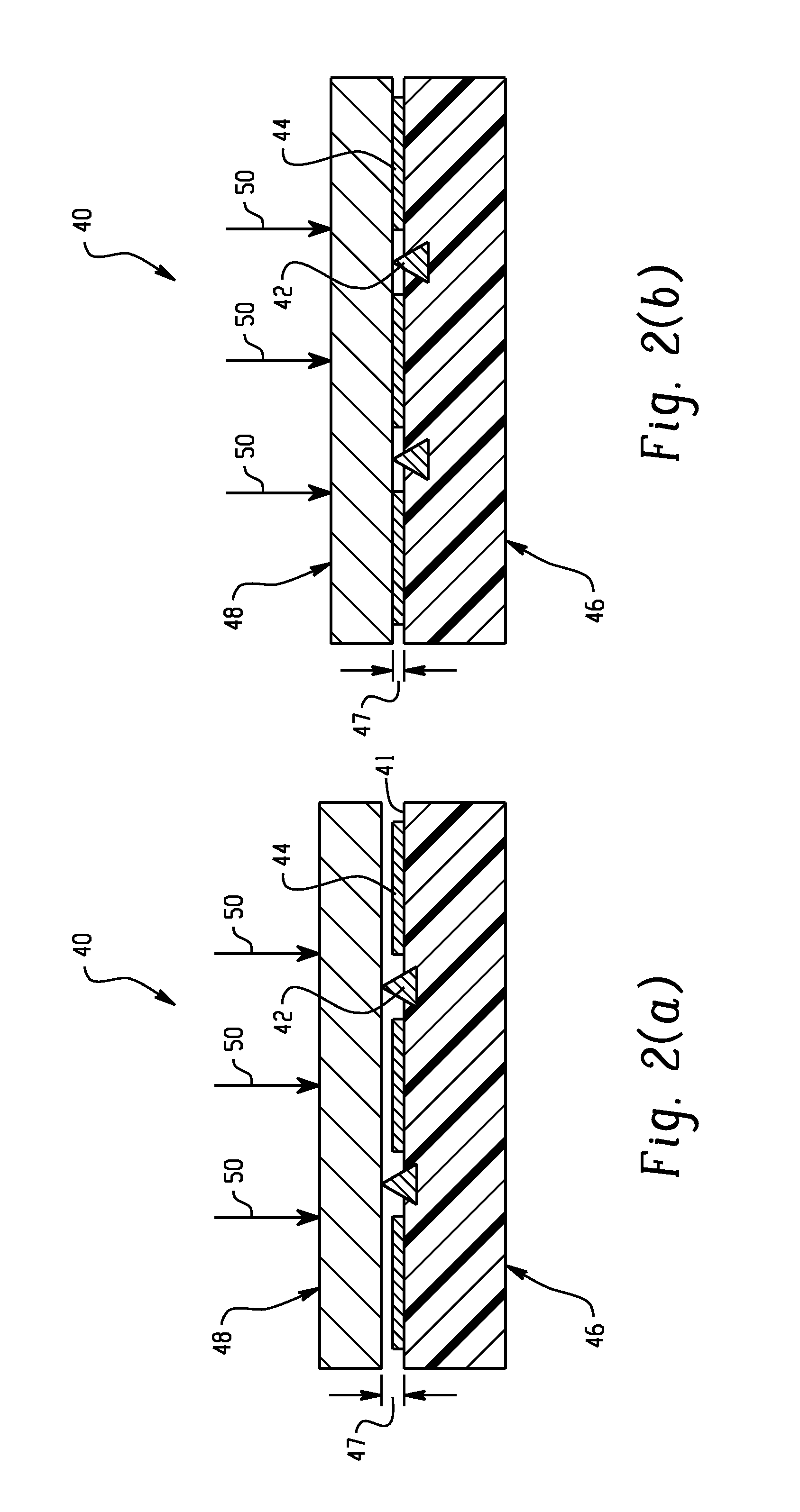
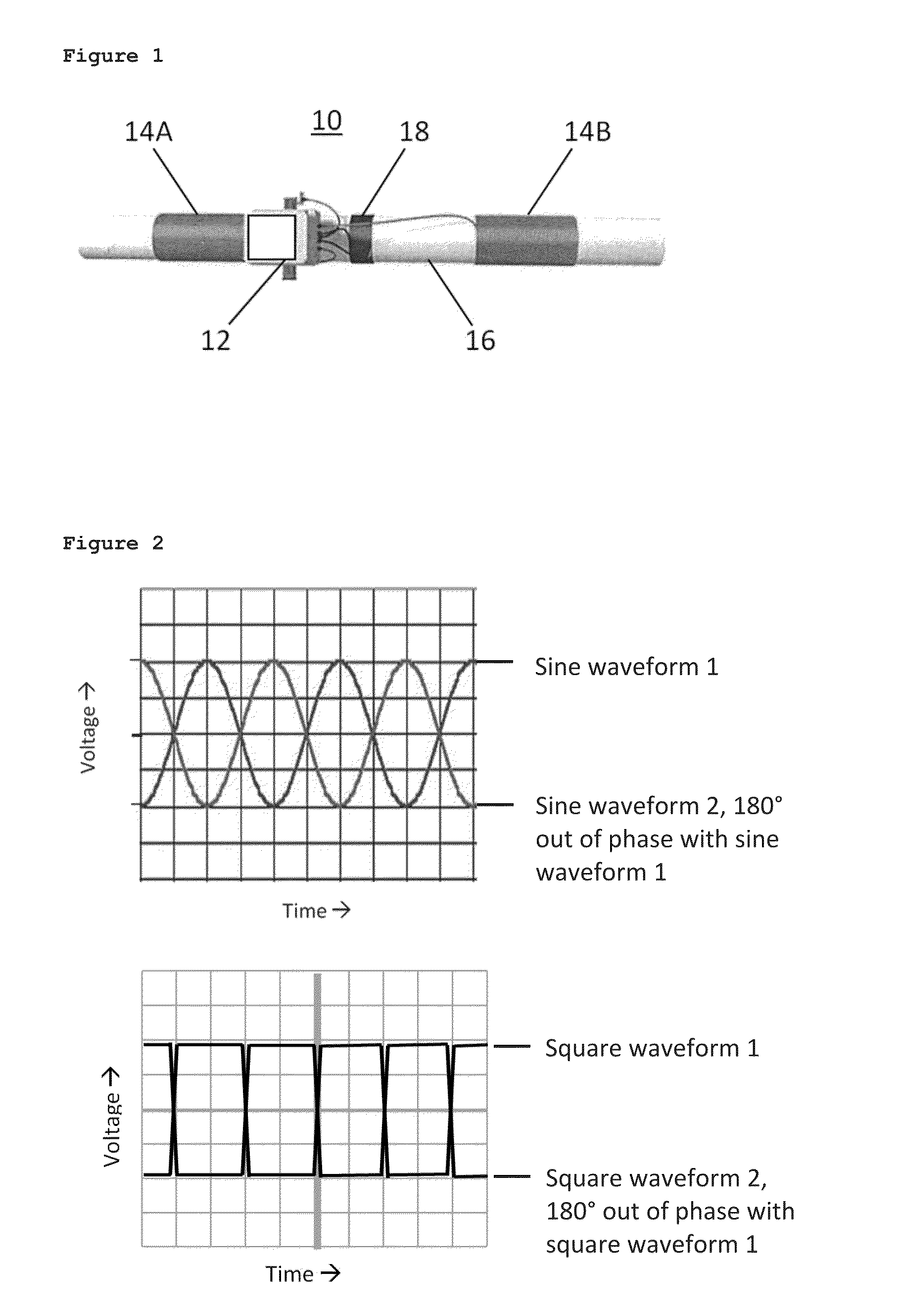
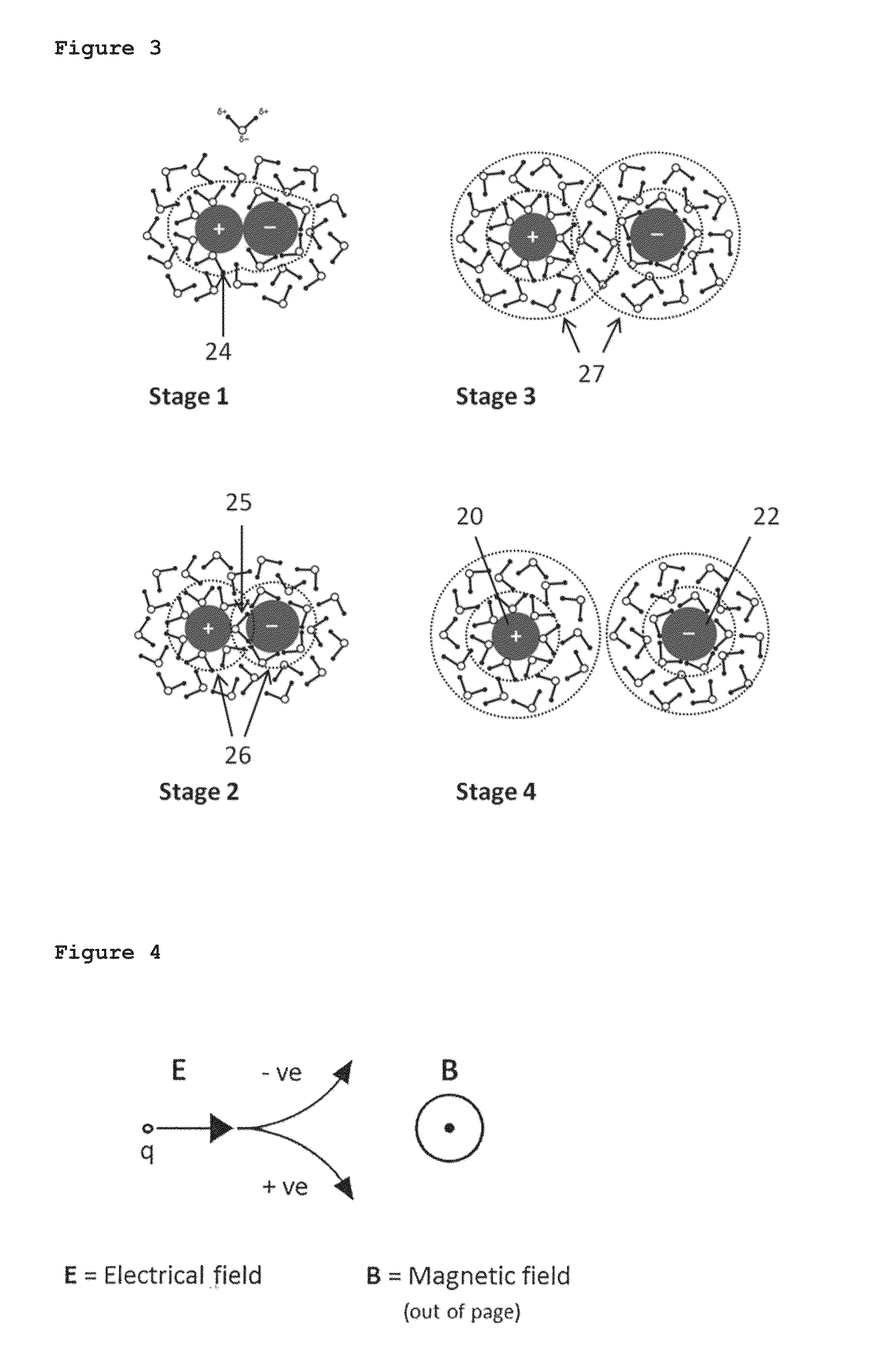
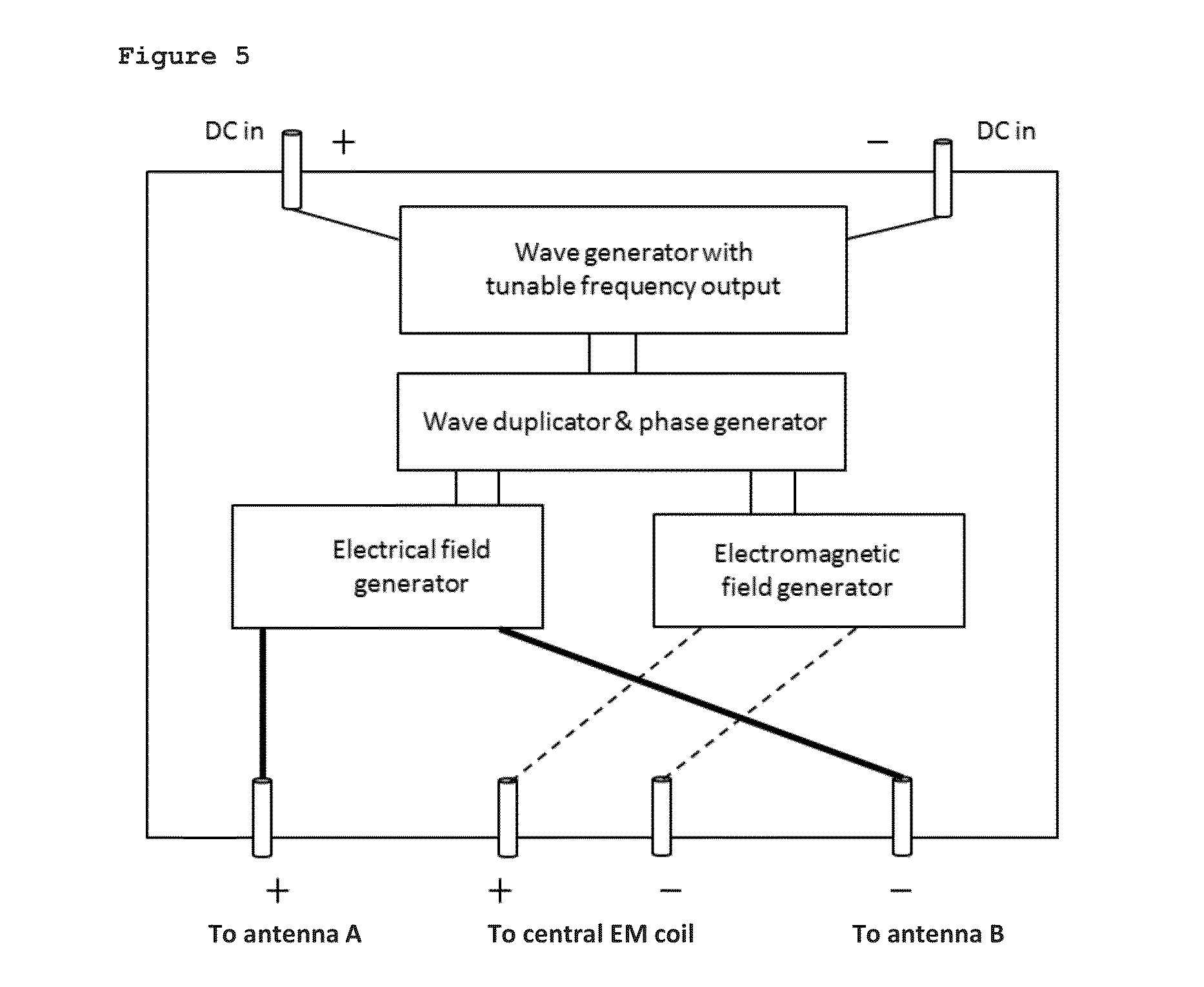
ActiveUS20140374236A1Improve solubilityImprove mobilityWater treatment parameter controlGeneral water supply conservationEngineeringSolvent
A liquid treatment device comprising: two antennae; an enclosure for holding a liquid including a solvent and a solute; a generator operatively connected to the two antennae to generate an oscillating voltage in each antenna, wherein each voltage is out of phase with the other to create an oscillating electric-field; and the liquid in the enclosure being subjected to the electric-field in the presence of a magnetic field to change the chemical and / or physical properties of the solute, without the liquid contacting the two antennae.
Owner:HYDROSMART
Electrochemical oxidation of hydrogen sulfide
The invention relates to a process for gas phase electrochemical oxidation of H2S to sulfur and water or steam using an electrolysis cell having an anode chamber on one side of a solid proton conducting membrane and a cathode chamber on the other side of the membrane. The process comprises the steps of passing H2S-containing gas through the anode chamber to contact a catalytic anode, where it reacts to produce elemental sulfur, protons and electrons. The protons pass through the membrane from the anode chamber to the cathode chamber. An oxygen-containing gas is passed through the cathode chamber to contact the catalytic cathode, where it reacts with protons and electrons to produce water or steam. During the process, both the anode chamber and cathode chamber are maintained at a temperature of at least 120° C. and an elevated pressure sufficient to keep the membrane moist. Sulfur is obtained in liquid or vapor form and is removed from the anode chamber while water or steam is removed from the cathode chamber. An electric current can be withdrawn from the anode and cathode. The cell can also be operated in the electrolysis mode to produce sulfur and hydrogen.
Owner:ETHYL TECH
Solid state surface catalysis reactor
InactiveUS6916451B1Physical/chemical process catalystsPhotovoltaicsChemical reactionElectric generator
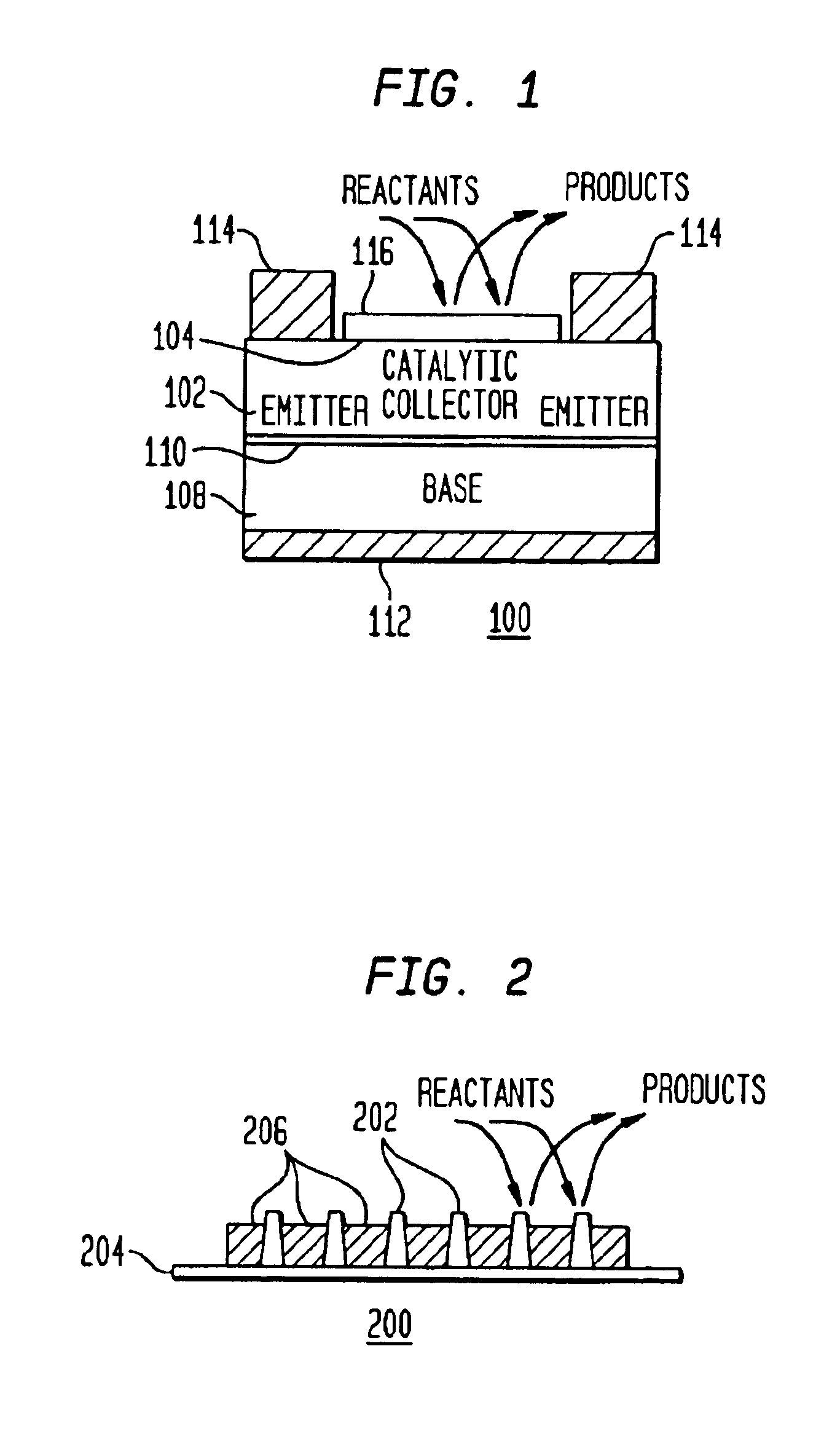
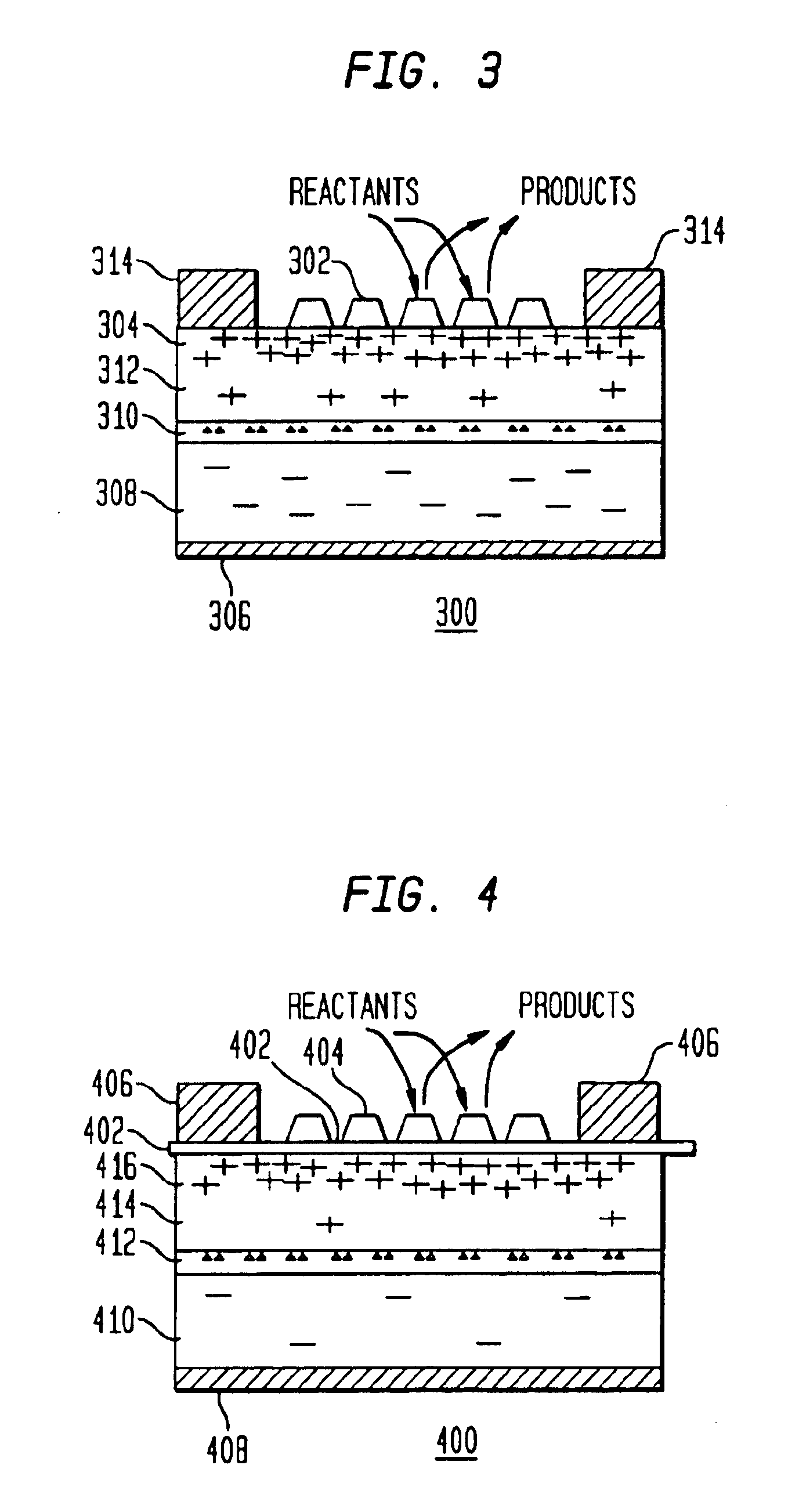
A method and apparatus to stimulate chemical reactions on a catalyst surface using electricity, and the reverse process to convert energy released from chemical reactions into electricity. A reversible emitter generates electrons which are injected into reactants on the catalyst surface, exciting chemically reactive vibrational resonances. Hot electrons created in the emitter region of a semiconductor junction diffuse to the co-located collector region and catalyst surface. Catalyst clusters or films bonded on the collector surface transfer the hot electrons to or from the catalyst surface having adsorbed reactants. The dimension of the catalyst-collector is less than the energy mean free path of hot electrons. The hot electrons excite reactant vibrations before thermalizing with the substrate lattice thereby accelerating reactions. The hot electrons are also generated by the same reactions on a catalyst surface. The method and apparatus is reversible and may be operated as an electric generator using chemical reactions.
Owner:NEOKISMET L L C
Projection display apparatus and system
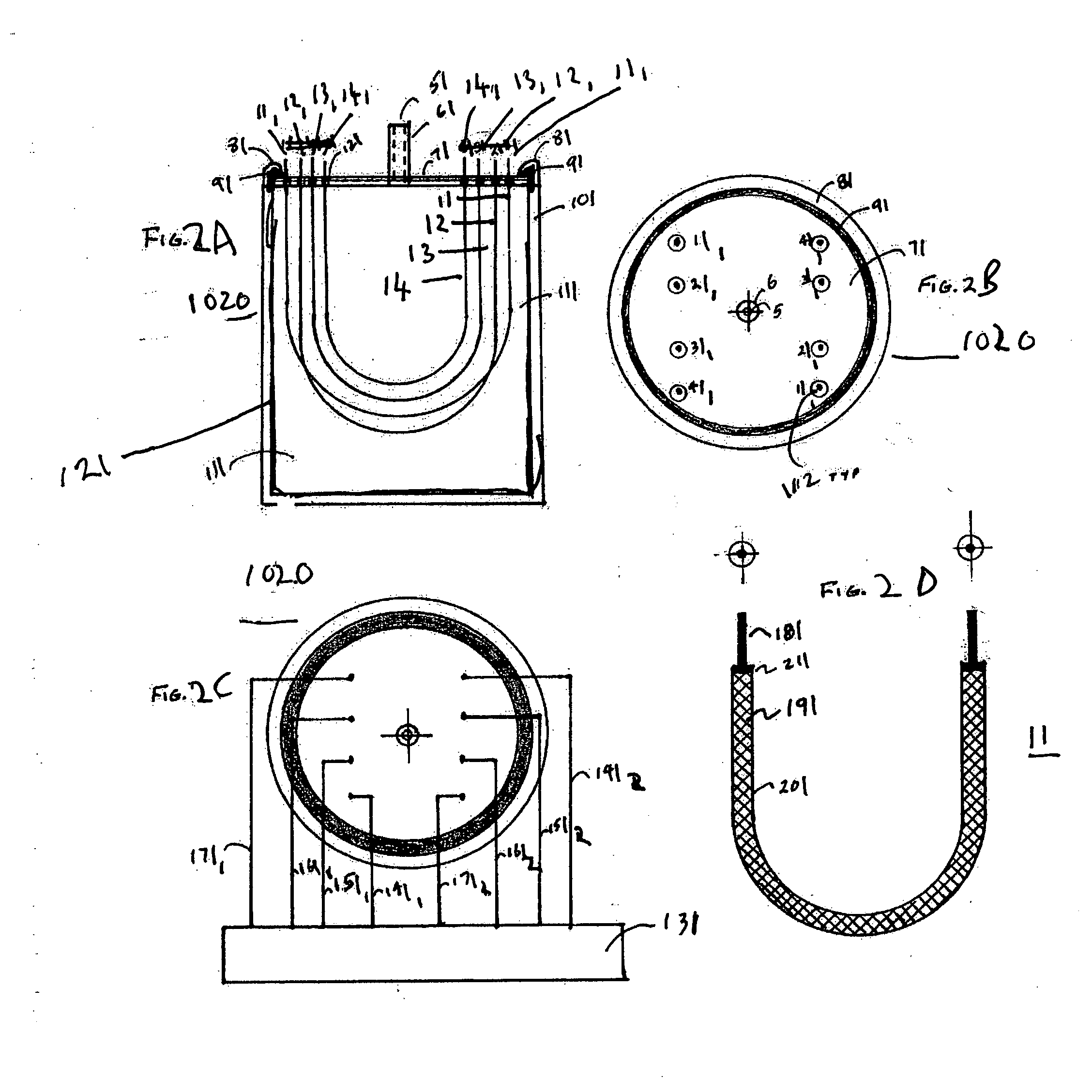
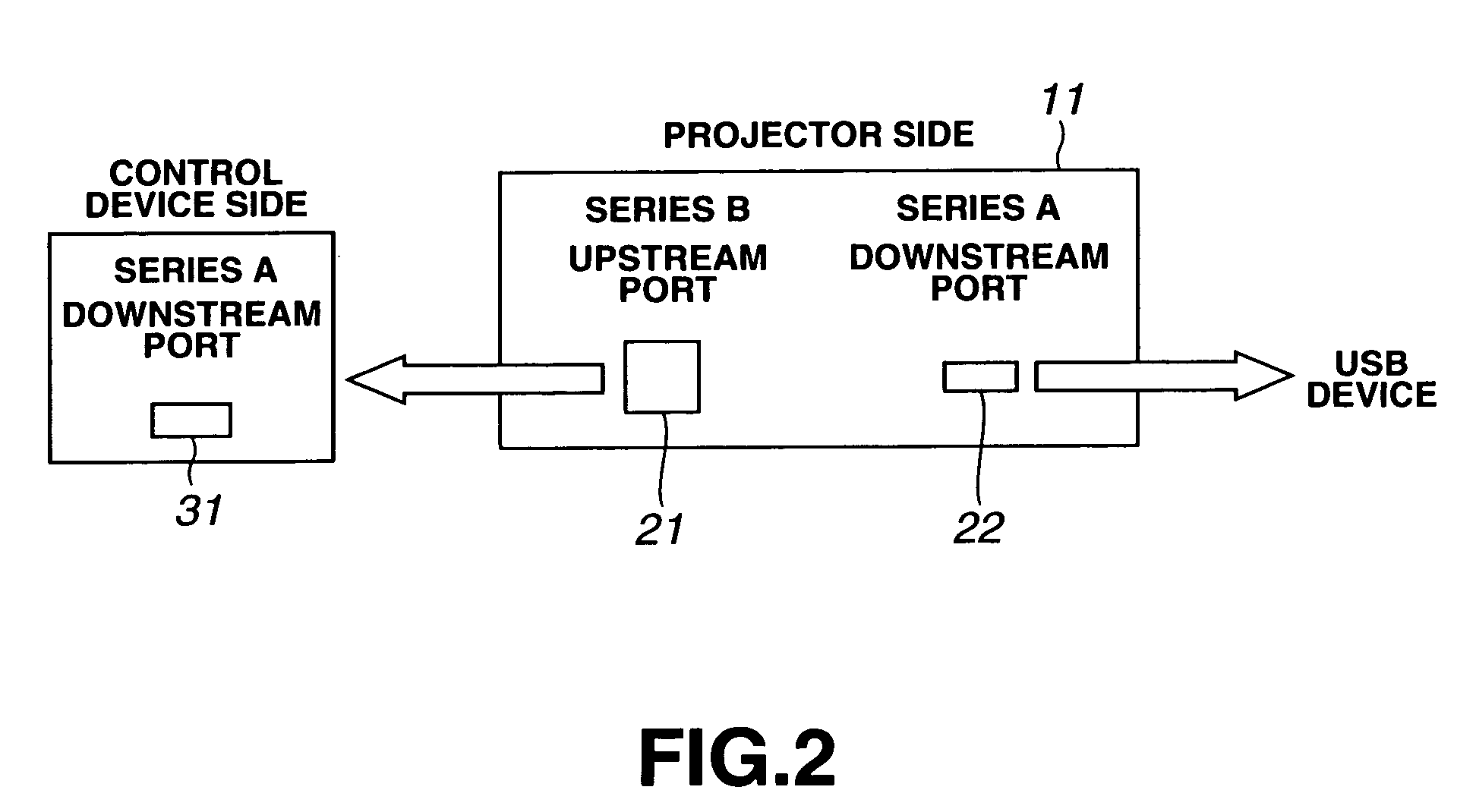
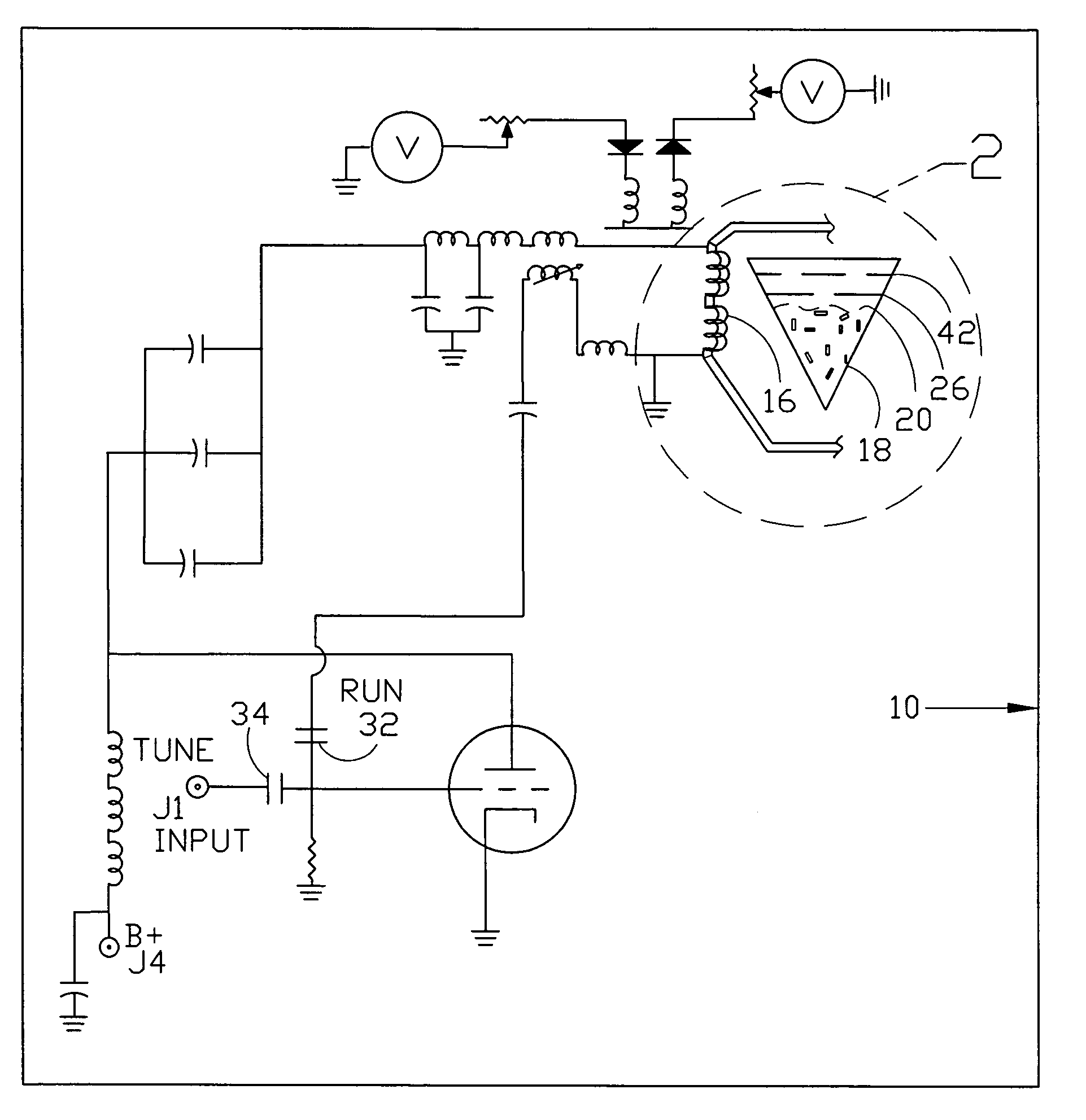
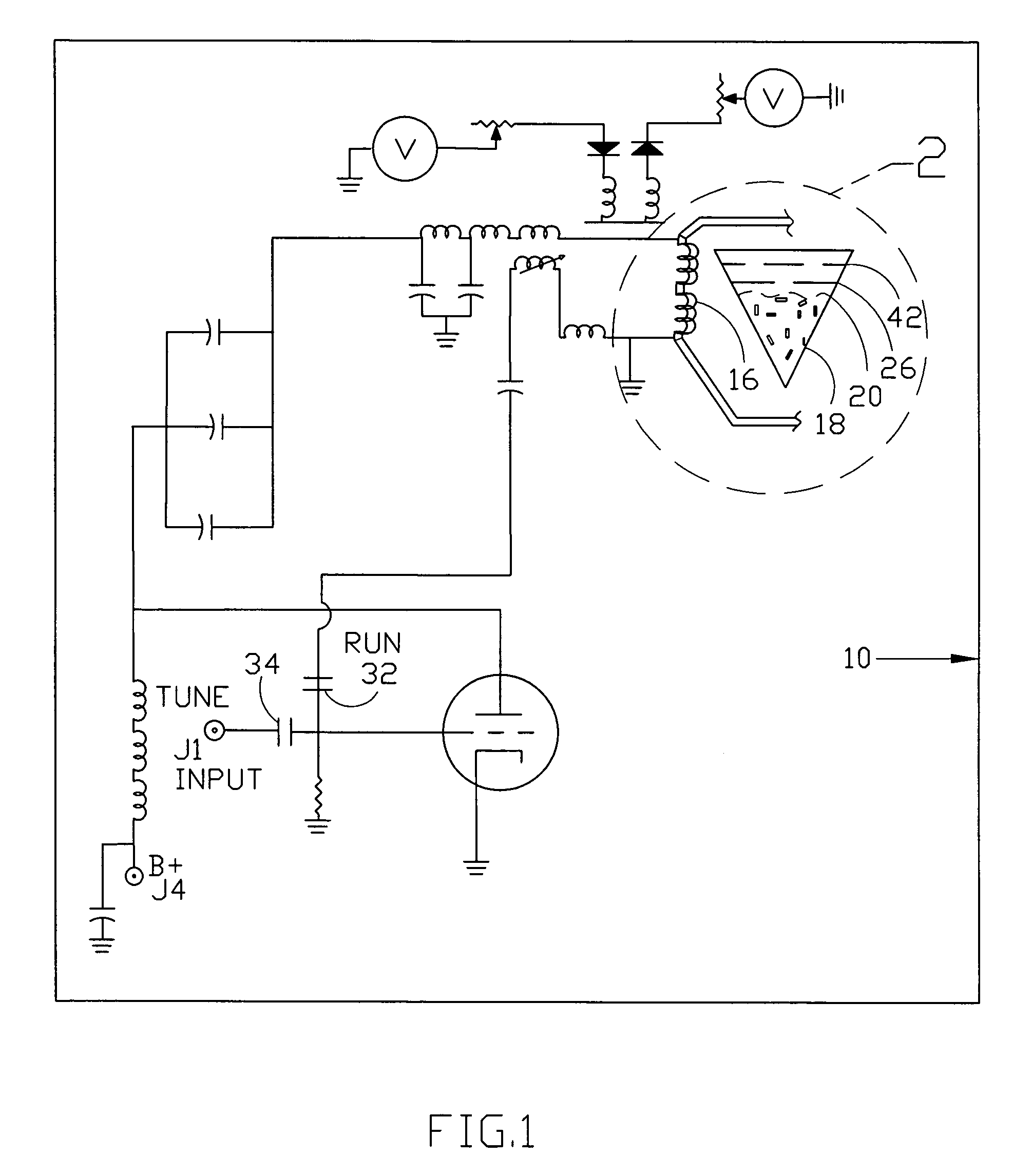
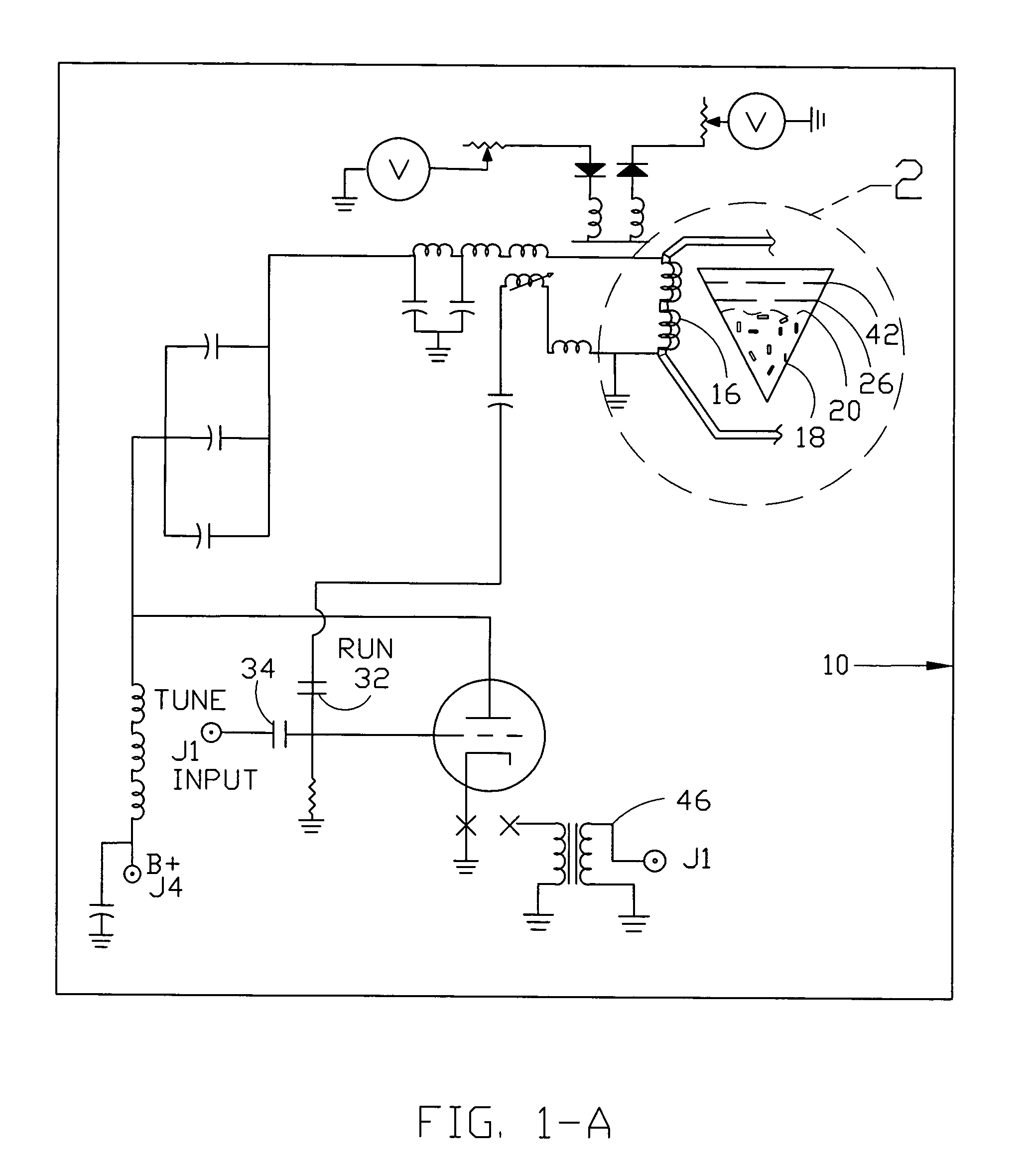
InactiveUS7061477B1Easy to operateImprove system performanceCathode-ray tube indicatorsElectrogenerative processesControl systemControl signal
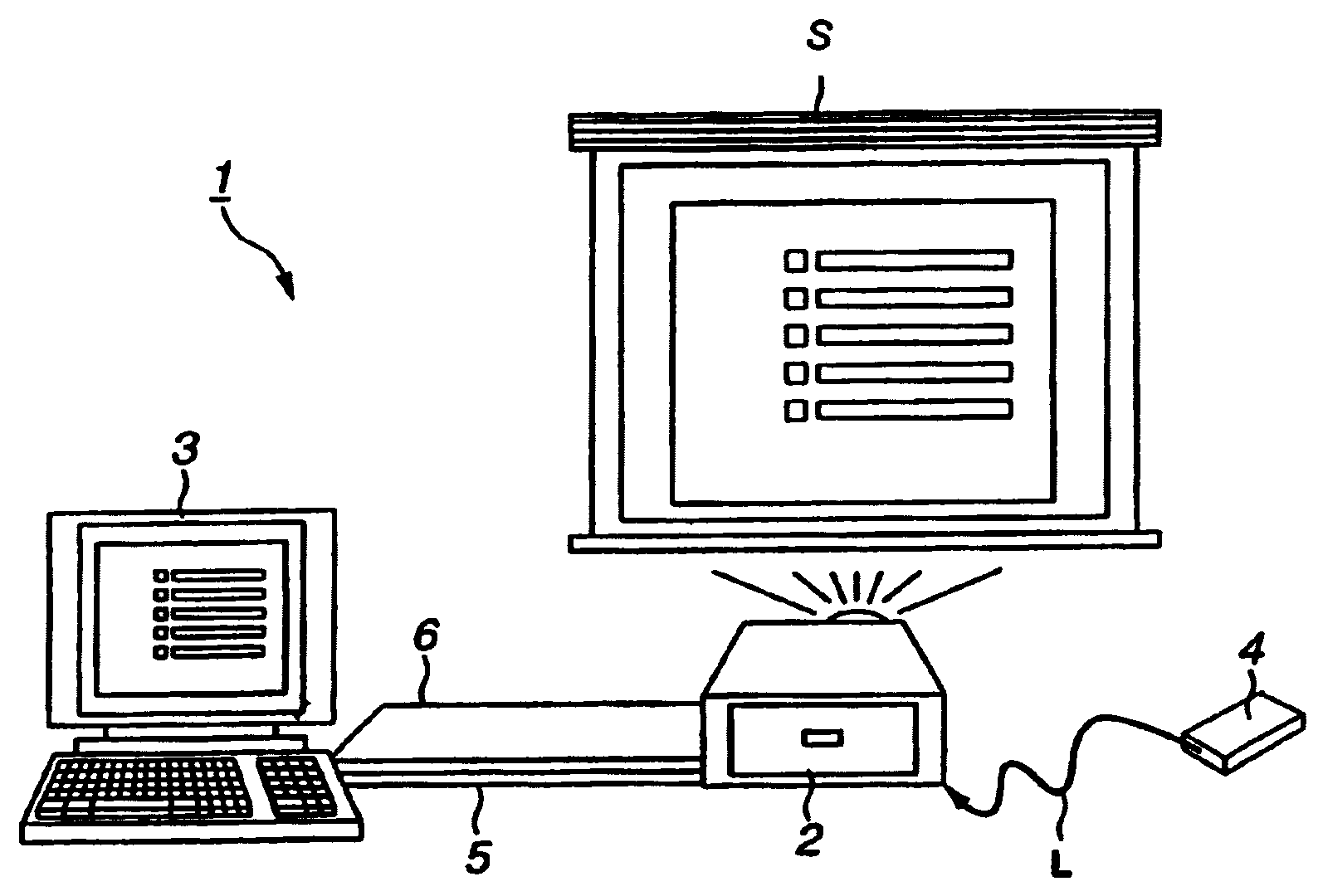
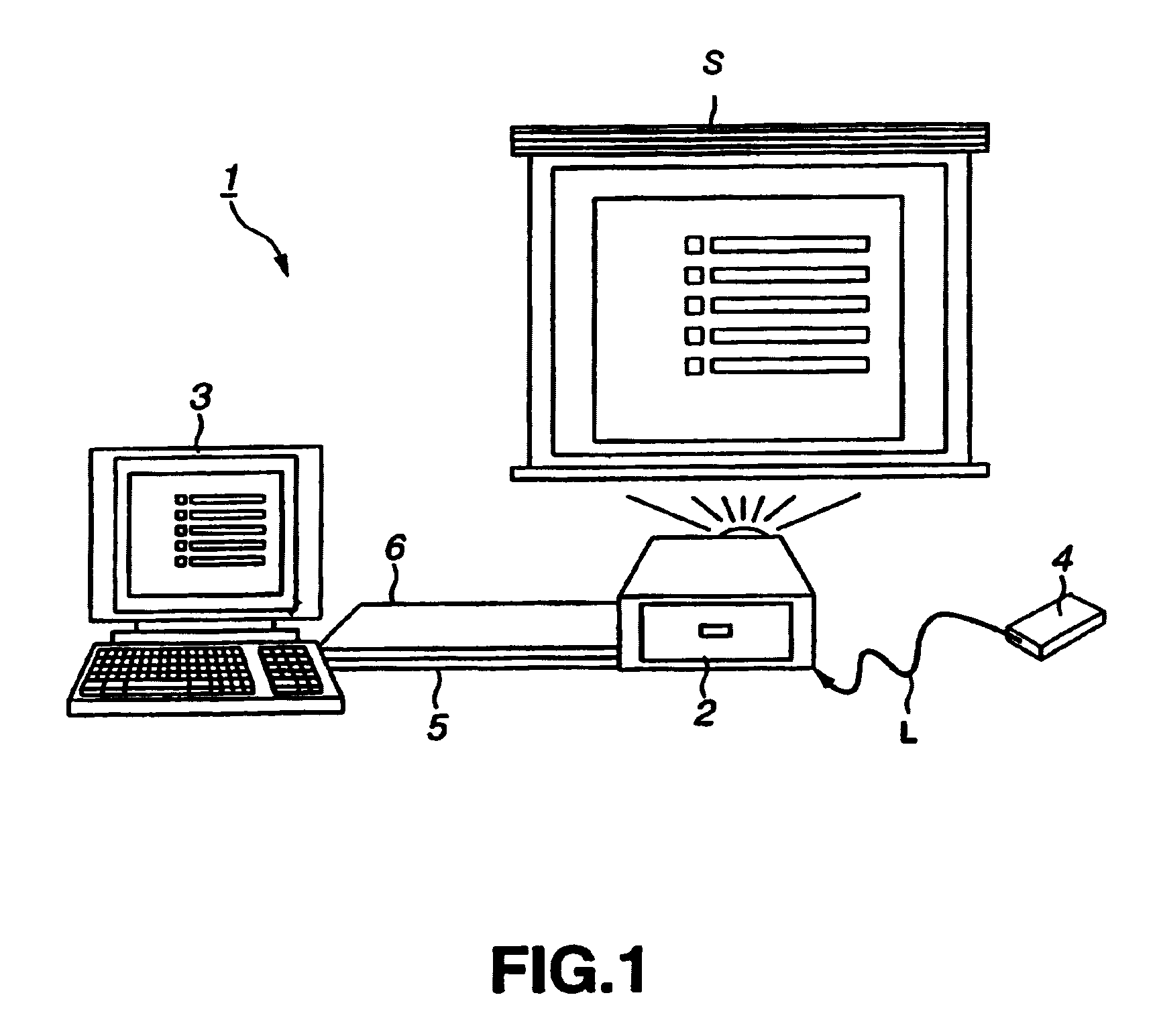
An image controlling system in which an image to be displayed is controlled by a control device is to be improved in its operating performance. A control device 3 and a projector 2 are interconnected over a USB cable 5 and an RGB cable 6 to make bi-directional data transmission / reception to display a picture by the projector 2 on a display screen S. The projector 2 includes a projection display unit 18 fed with display data from the control device 3 to project light to display a picture represented by the display data, a hub 11 connected to the control device 3 and to a USB device to make data input / output based on the supplementary information and a display controller19 for controlling a picture demonstrated by the projection display unit 18 based on a display control signal generated by the control device 3. The control device includes a connector 32, connected to the projector 2, and a controller 33 for outputting the control signal and the display data to have a picture displayed by the projector 2.
Owner:SONY CORP
Cooling medium flow passage
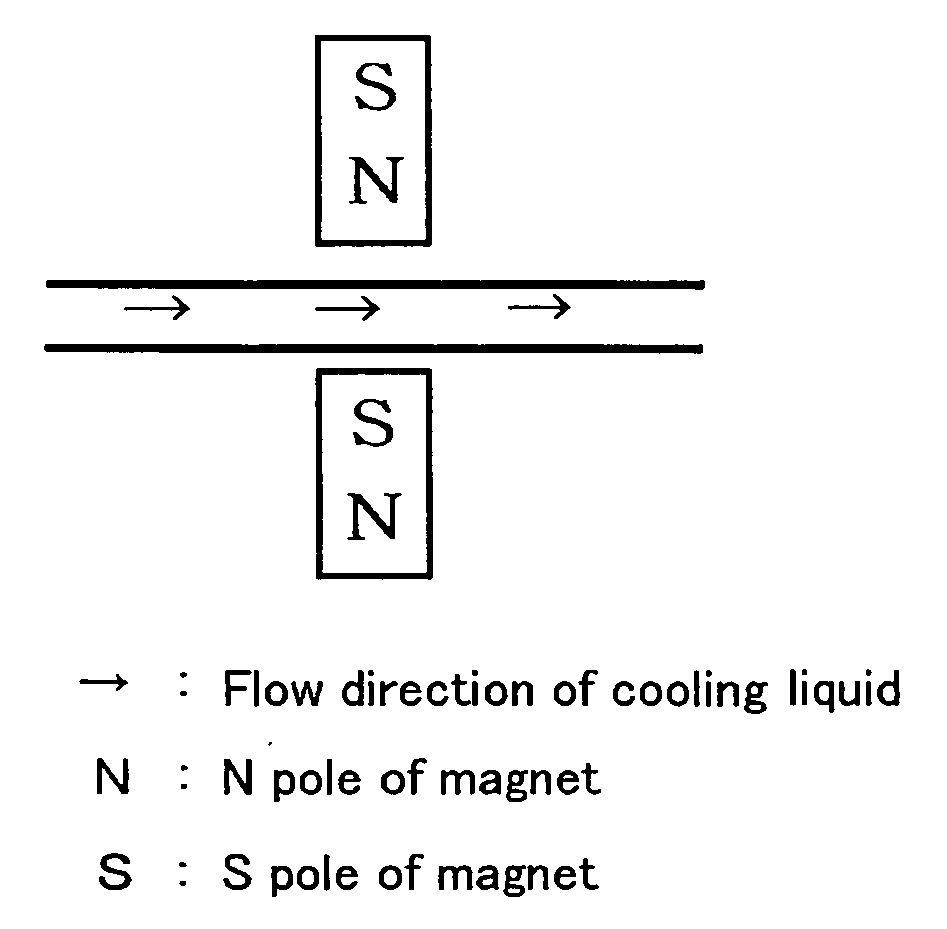
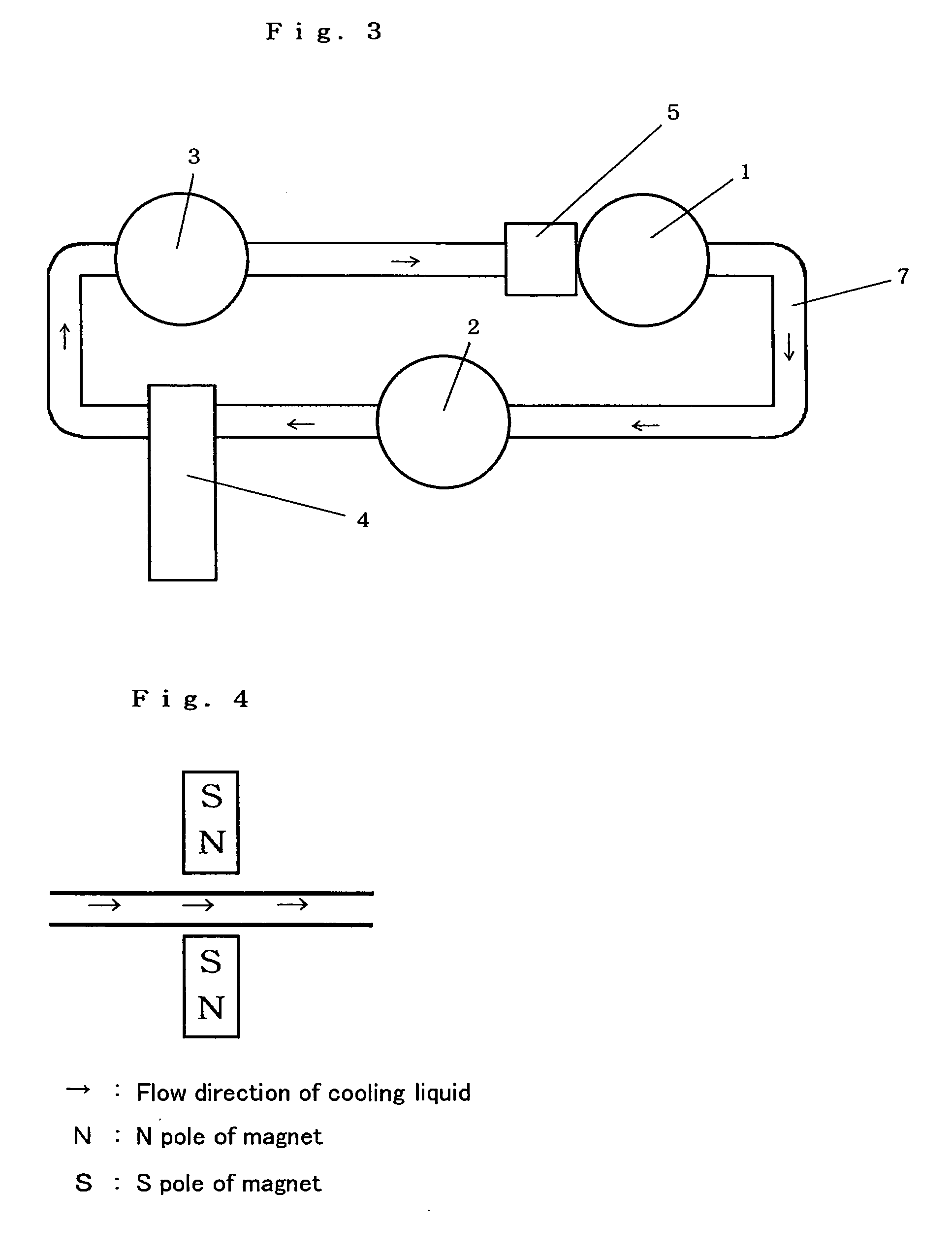
InactiveUS20070020109A1Avoid cloggingAvoid contaminationPump componentsScale removal and water softeningLiquid cooling systemEngineering
The present invention provides a cooling medium flow path for improving cooling efficiency of a cooling medium used for liquid-cooling systems for motors, radiators and the like. The cooling medium flow path according to the present invention is capable of increasing cooling efficiency of a cooling medium by providing magnetic members for generating a magnetic force in a direction substantially perpendicular to the flow direction of the cooling medium so that clusters of a liquid, such as cooling water, antifreeze liquid or the like flowing through the flow path may be finely divided or activated.
Owner:KANKYOKIKI CORP
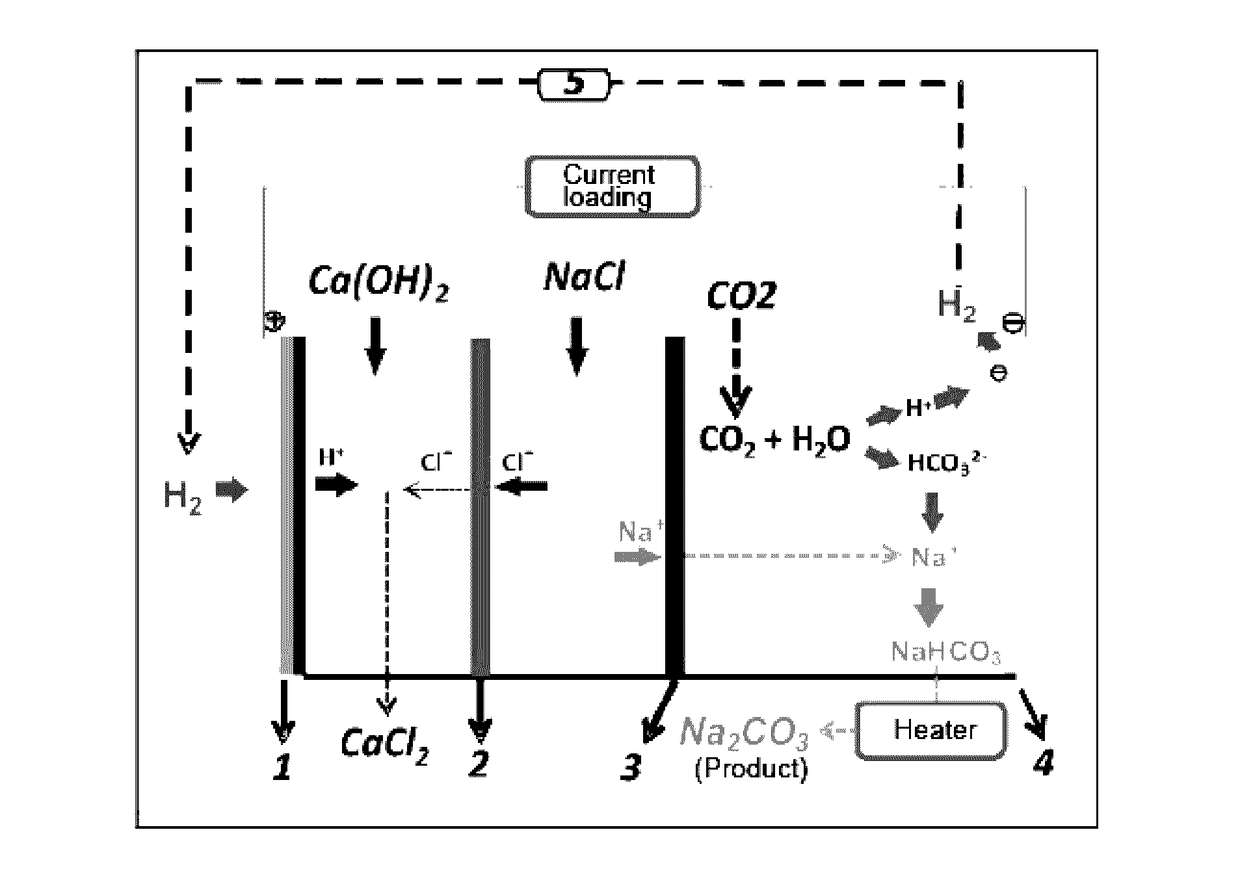
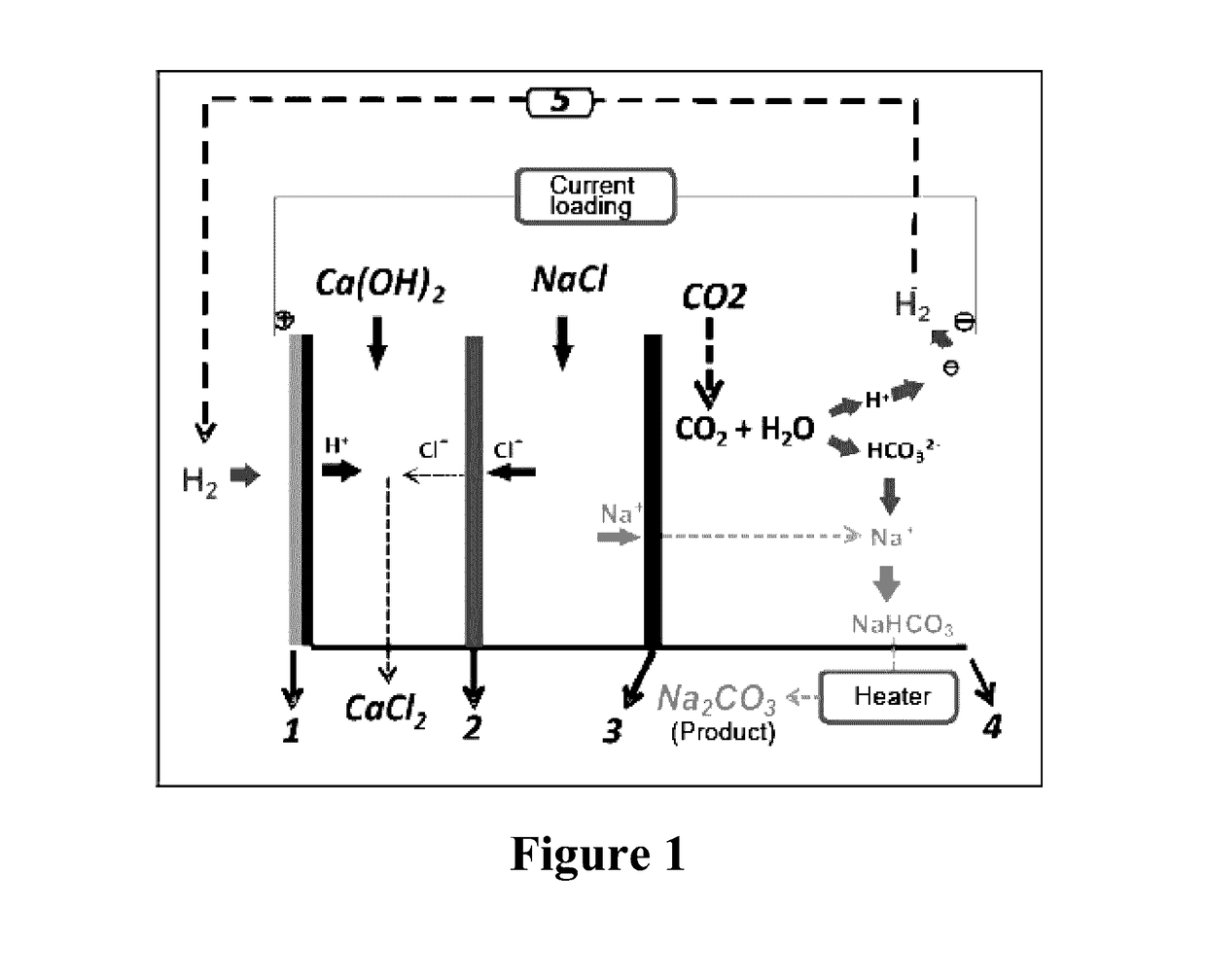
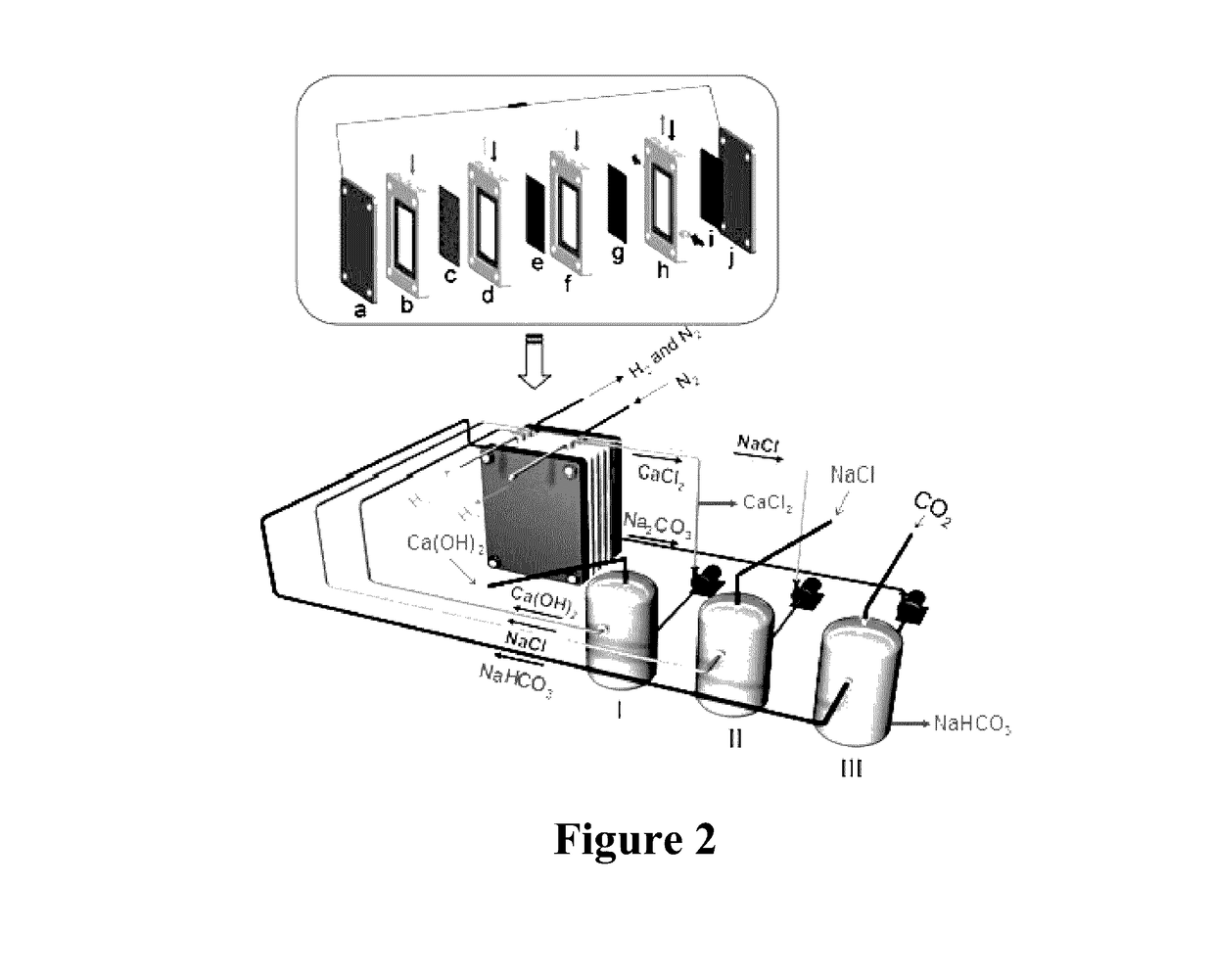
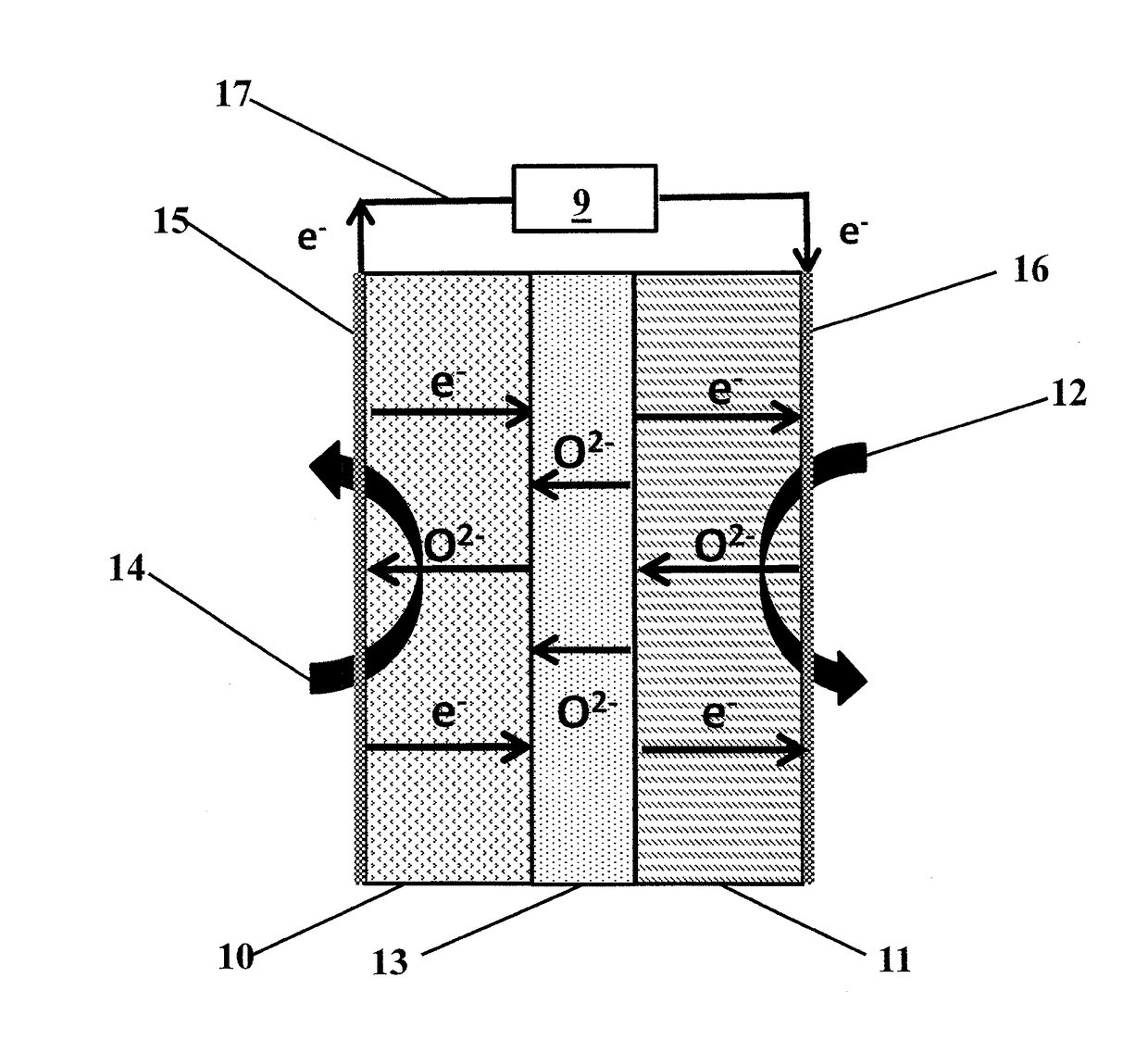
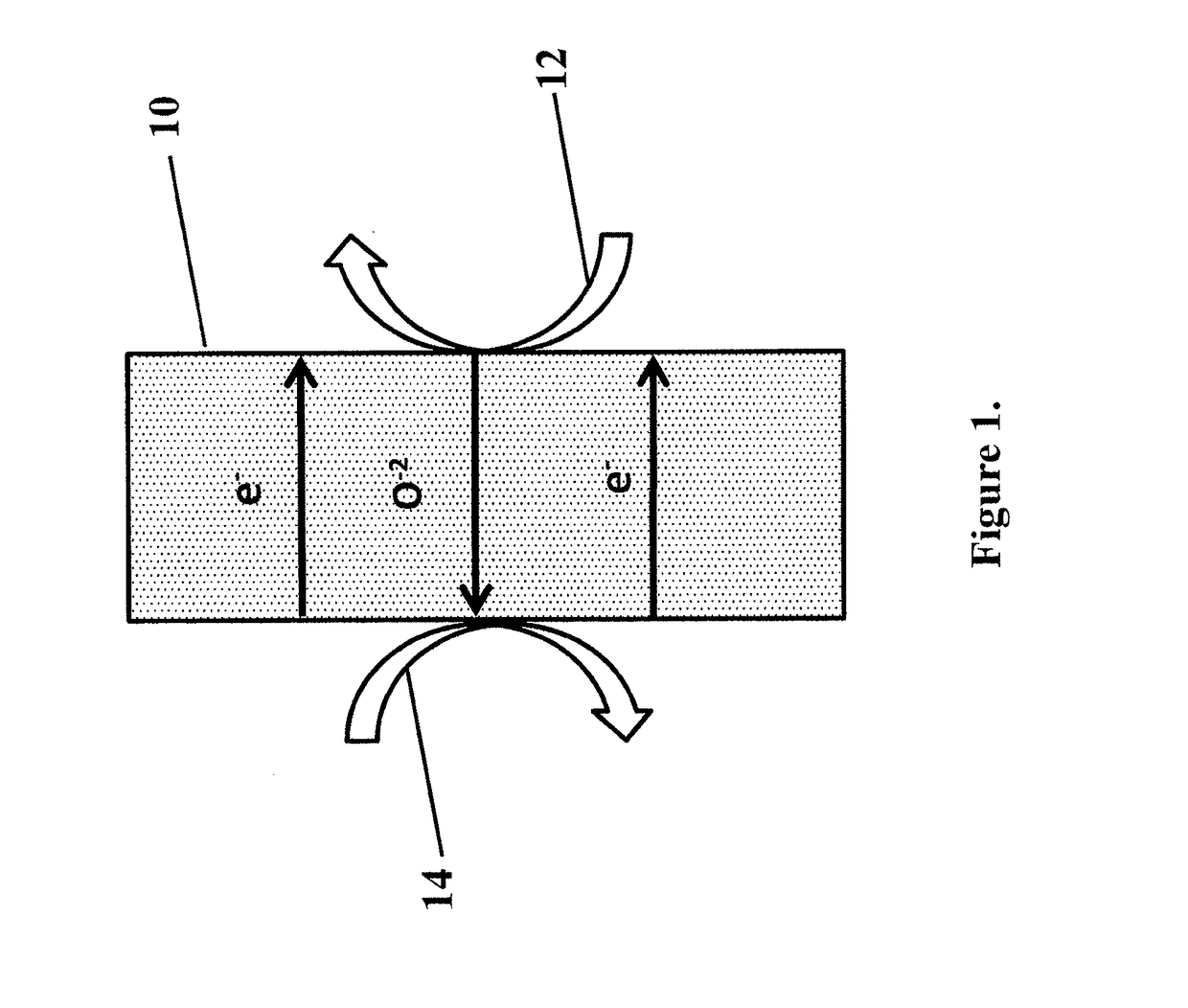
Method and device for using co2 mineralization to produce sodium bicarbonate or sodium carbonate and output electric energy
ActiveUS20170137951A1Facilitate spontaneous reaction and separationImprove conductivityCellsCell electrodesSodium bicarbonateElectrolysis
Disclosed are a method and device for using CO2 mineralization to produce sodium bicarbonate or sodium carbonate and output electric energy. The device comprises an anode area, an intermediate area, and a cathode area. The anode area and the intermediate area are spaced by a negative ion exchange membrane (2). The intermediate area and the cathode area are spaced by a positive ion exchange membrane (3). The anode area, the intermediate area, and the cathode area can accommodate corresponding electrolytes. An anode electrode (1) is disposed in the anode area, a cathode electrode (4) is disposed in the cathode area, and the cathode electrode and the anode electrode are connected through a circuit. A raw material hydrogen gas inlet is disposed in the anode area, and a CO2 inlet and a product hydrogen gas outlet are disposed in the cathode area. The method is based on the principle of CO2 mineralization and utilization, combines the membrane electrolysis technology, facilitates spontaneous reaction by using the acidity of CO2 and the alkalinity of the reaction solution and realizes separation of the products, and converts through a membrane electrolysis apparatus the energy released by the reaction into electric energy at the same time when producing the sodium bicarbonate or sodium carbonate and outputs the electric energy. The method and device have low energy consumption, high utilization rate of raw materials and little environmental pollution, and can output electric energy while producing sodium carbonate at the same time.
Owner:SICHUAN UNIV
Microwave-based conveying devices and processing of carbonaceous materials
InactiveUS20100230270A1Liquid hydrocarbon mixture productionEnergy based chemical/physical/physico-chemical processesSolid carbonMicrowave
Owner:GORTRAGH DEV
Reactor, process, and system for the oxidation of gaseous streams
InactiveUS20170247803A1Semi-permeable membranesElectrolysis componentsElectrical conductorLiquid fuel
A reactor and process capable of concurrently producing electric power and selectively oxidizing gaseous components in a feed stream, such as hydrocarbons to unsaturated products, which are useful intermediates in the production of liquid fuels. The reactor includes an oxidation membrane, a reduction membrane, an electron barrier, and a conductor. The oxidation membrane and reduction membrane include an MIEC oxide. The electron barrier, located between the oxidation membrane and the reduction membrane, is configured to allow transmission of oxygen anions from the reduction membrane to the oxidation membrane and resist transmission of electrons from the oxidation membrane to the reduction membrane. The conductor conducts electrons from the oxidation membrane to the reduction membrane.
Owner:ECOCATALYTIC INC
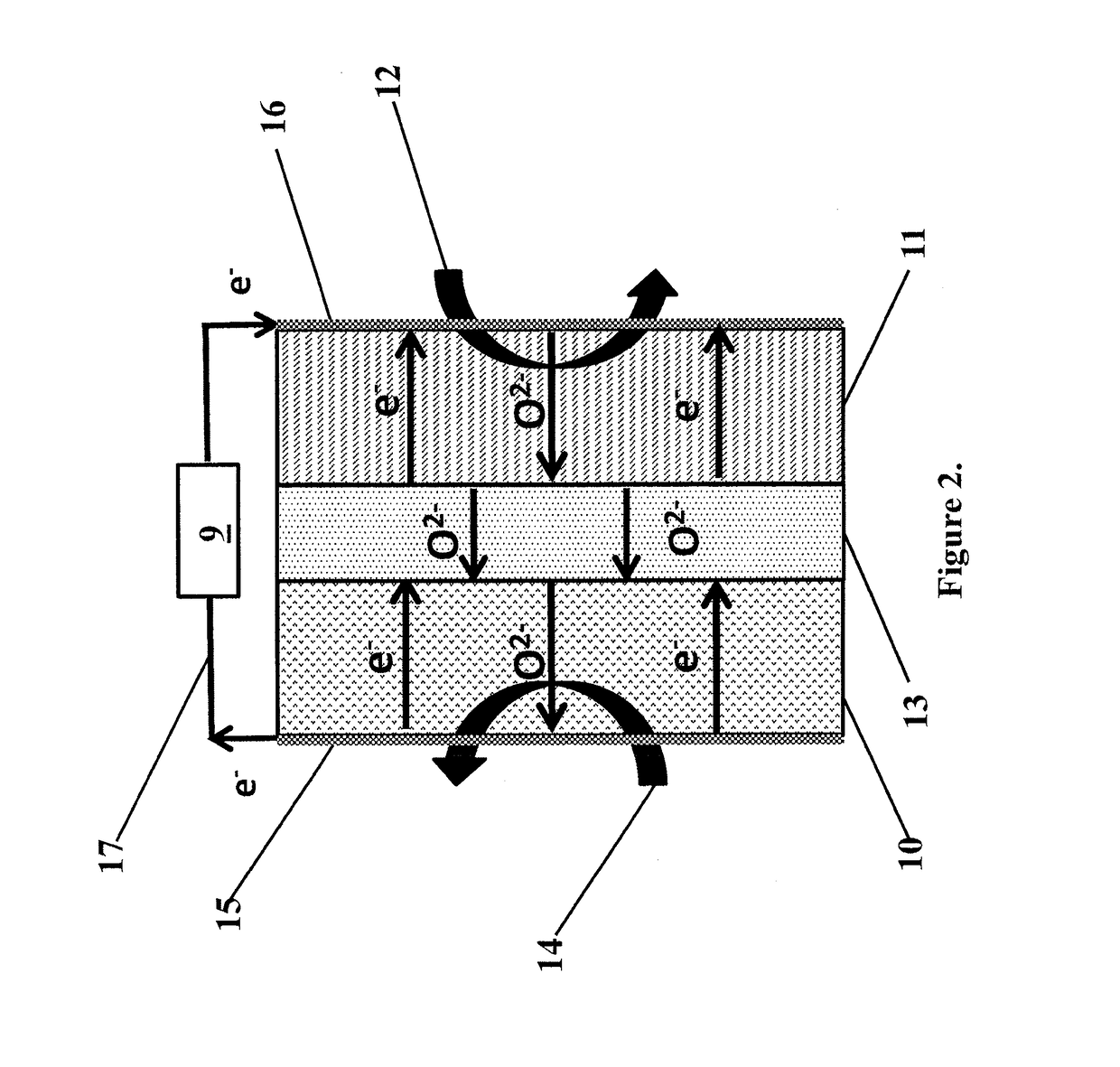
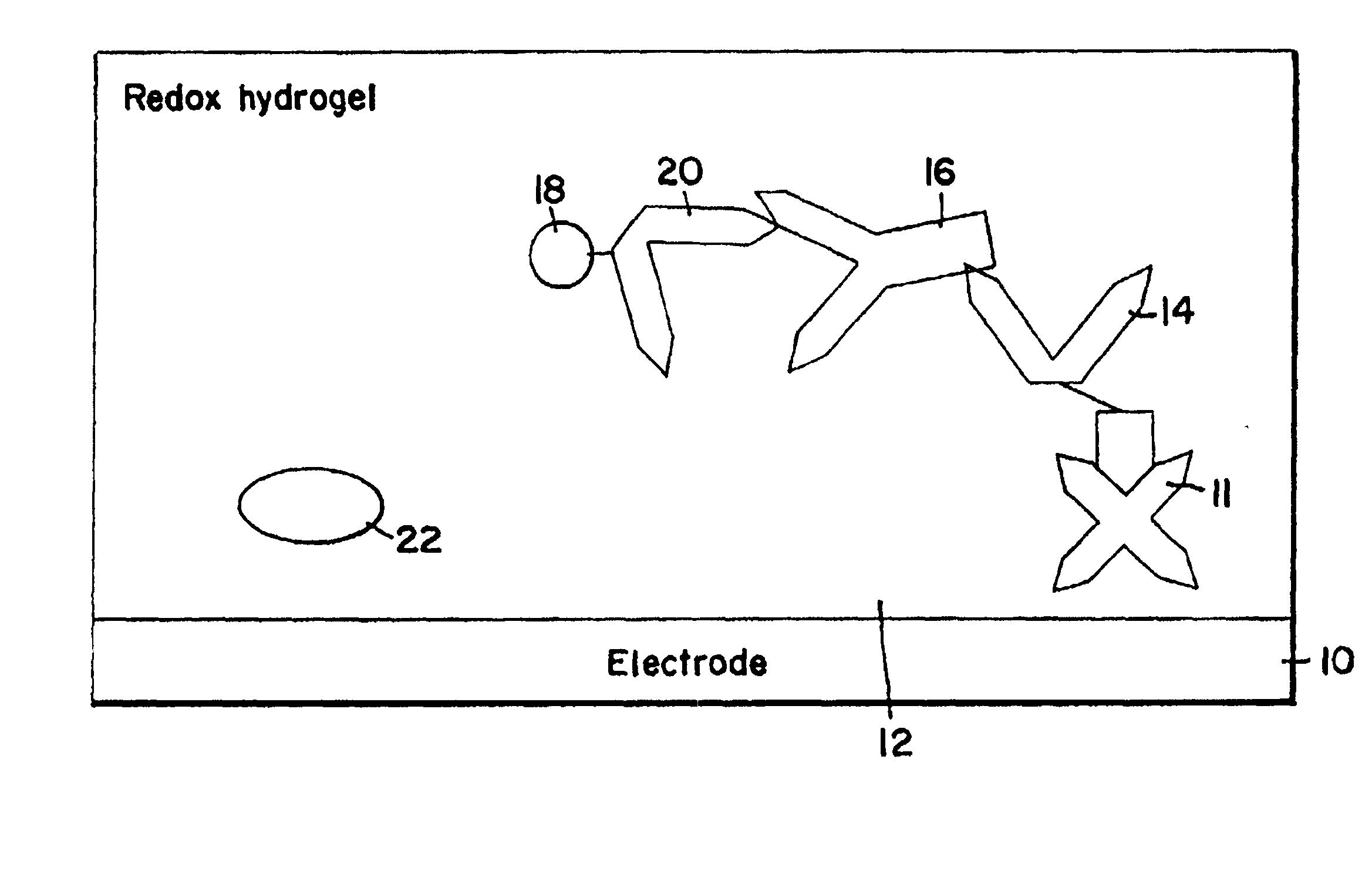
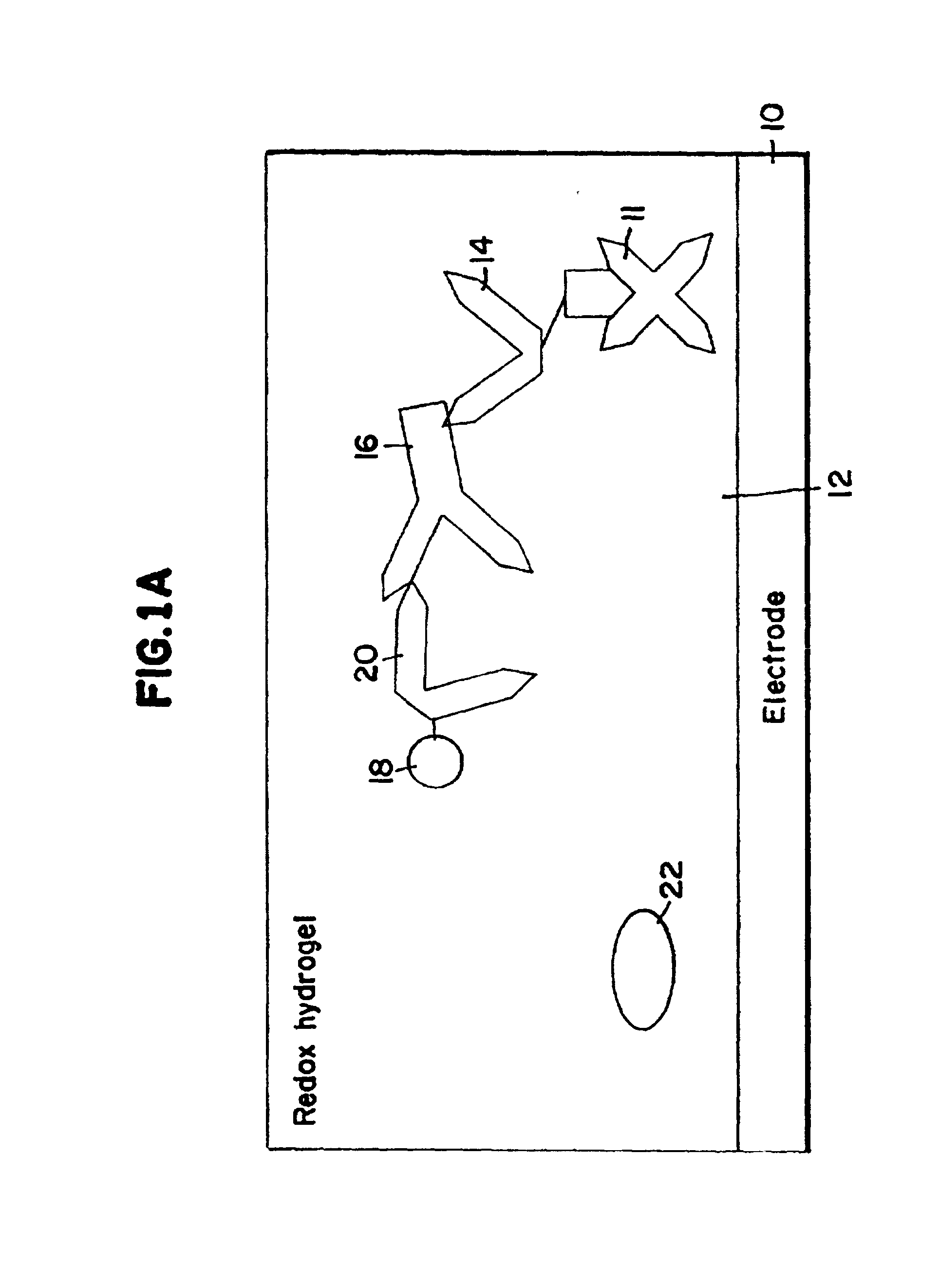
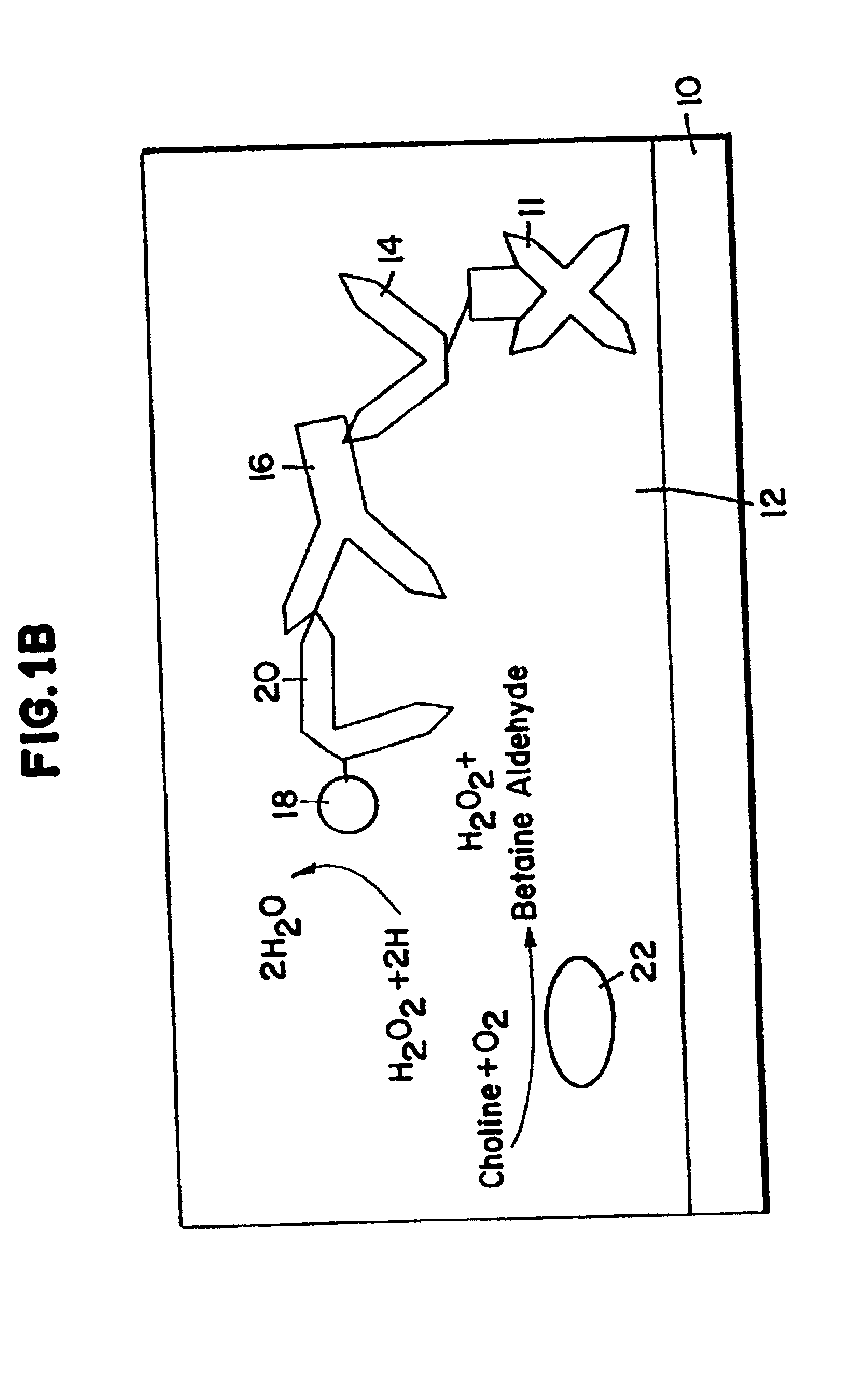
Electrochemical affinity assay
InactiveUS20020137193A1Bioreactor/fermenter combinationsBiological substance pretreatmentsPhysical chemistryAssay
Owner:ABBOTT DIABETES CARE INC +1
Apparatus for retaining magnetic particles within a flow-through cell
InactiveUS20050208464A1Efficient captureIncrease the areaBioreactor/fermenter combinationsSludge treatmentFlow through cellEngineering
An apparatus for retaining magnetic particles within a segment of a flow-through cell during flow of a fluid through the cell comprises (a) optionally, an electrical current source; (b) an electromagnet having a winding connected to the current source and an air gap between at least one pair of poles each of which has a corrugated outer surface and (c) a flow-through cell which is configured and dimensioned to receive an amount of magnetic particles to be retained within the flow-through cell and to allow flow of a liquid through the flow-through cell. The liquid carries molecules or particles to be captured by means of the magnetic particles. A portion of the flow-through cell is inserted in air gap.
Owner:ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL)
Electrochemical zinc-water hydrogen making and storing method
InactiveCN1854063AImprove controllabilityEasy to startHydrogenElectrolysis componentsHydrogen fuel cellOxygen
A method for producing and storing hydrogen by electrochemical zinc-water is carried out by connecting electrode and zinc electrode external circuit when releasing hydrogen, water reduction reacting on electrode, releasing hydrogen, zinc oxidation reacting on zinc electrode, generating zinc oxide, supplementing water to enclosed system, connecting power supply negative electrode onto zinc electrode external circuit, connecting power supply positive electrode onto electrode external circuit, inducing DC, zinc reduction reacting on zinc electrode, reducing zinc oxide into zinc, restoring zinc electrode, oxidation reacting, generating oxygen and discharging. It is an enclosed system consisted of electrode electrolyte and zinc electrode, electrode and zinc are connected with external circuit. It is simple and can be used to provide hydrogen source and generation.
Owner:黄潮 +1
Method and device for measuring, calibrating and using laser tweezers
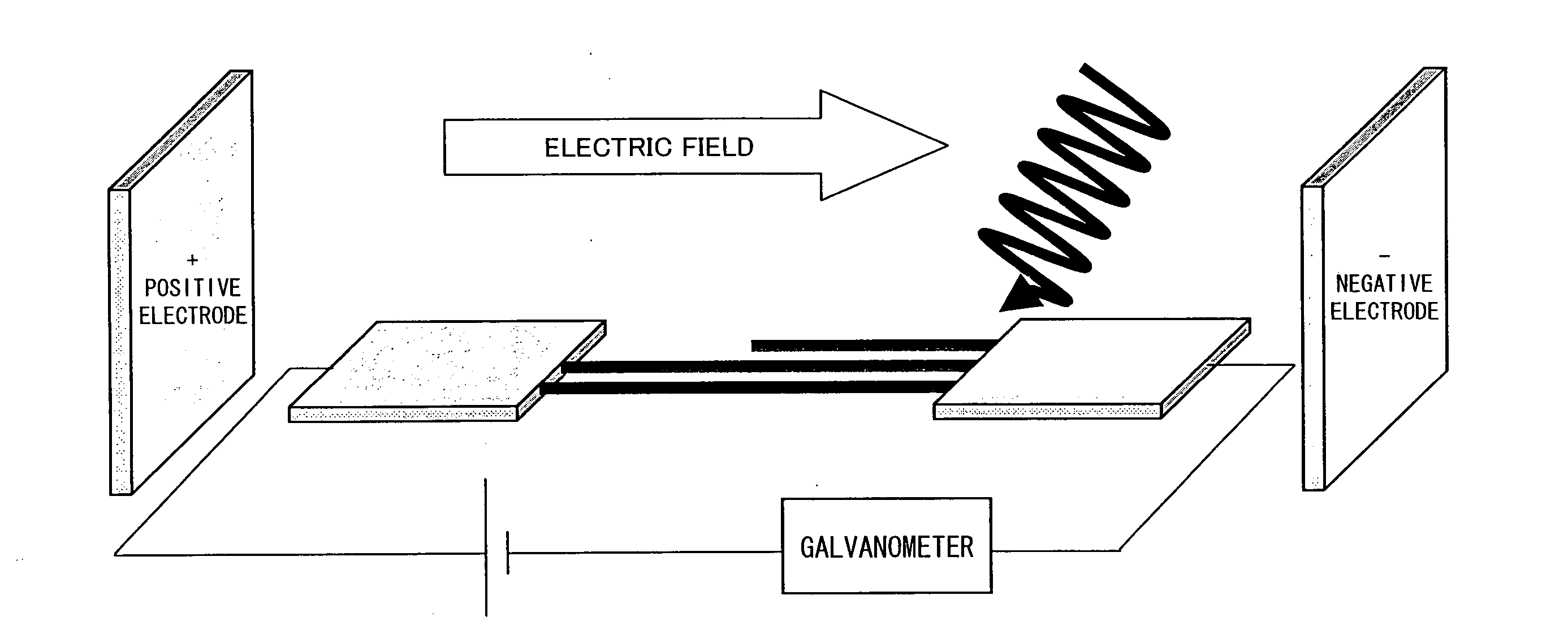
InactiveUS6991906B1Increase amplitudeAccurate thresholdImmobilised enzymesOptical radiation measurementElectricityLight beam
To measure or exert optically-induced forces on at least one particle in the focus of an optical cage, the following steps are taken:a) the focus is positioned in a microelectrode arrangement with a three-dimensional electrical field that has a field gradient which forms an electrical capture area, and the focus is at a distance from the capture are andb) the amplitude of the electrical field, the light power of the light beam forming the optical cage, and / or the distance of the capture area from the focus are varied to detect which varied field property moves the particle from the focus to the capture area or vice versa, or at least to temporarily move the particle into the capture area.
Owner:EVOTEC BIOSYST +1
Electrochemical Method for Producing and Storing Hydrogen by the Redox of Zinc and Water
InactiveUS20080190781A1Improve reliabilityLow costHydrogenElectricity cogenerationHydrogen fuel cellOxygen
Disclosed herein is an electrochemical method for producing and storing hydrogen, which is a closed system consisting of a gas-generating electrode, an electrolyte and a zinc electrode, the gas-generating electrode and zinc electrode are connected respectively to the external circuits; characterized in that switching on the external circuit of the gas-generating electrode and zinc electrode the hydrogen is to be released, the reduction reaction of water occurs on the gas-generating electrode producing hydrogen; zinc is oxidized on the zinc electrode generating the oxidation products of zinc; when the hydrogen is to be stored, supplementary water is supplied to the closed system, the negative pole of power source is connected to the external circuit of the zinc electrode, and the positive pole of power source is connected to the external circuit of the gas-generating electrode, switching on the direct current, the reduction reaction of zinc occurs on the zinc electrode, the oxidation products of zinc are reduced into zinc, renew the zinc electrode, the oxidation reaction of water occurs on the gas-generating electrode, the oxygen is generated and discharged. A simple and convenient process and widely applied are property of the present invention. It suits to provide hydrogen for hydrogen fuel cells. While providing the hydrogen, it can also generate electricity as secondary product, which generates the electricity together with a fuel cell.
Owner:HUANG CHAO
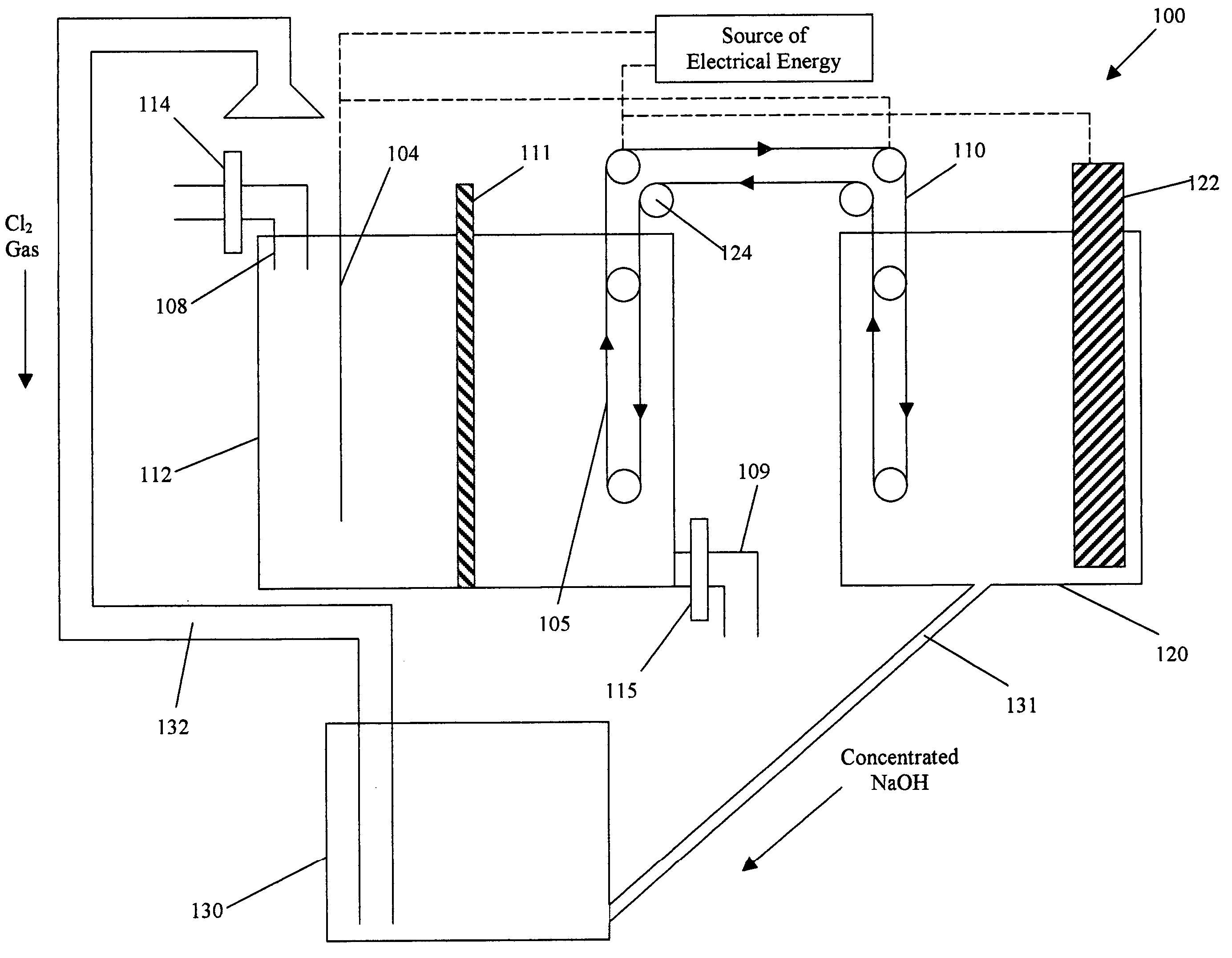
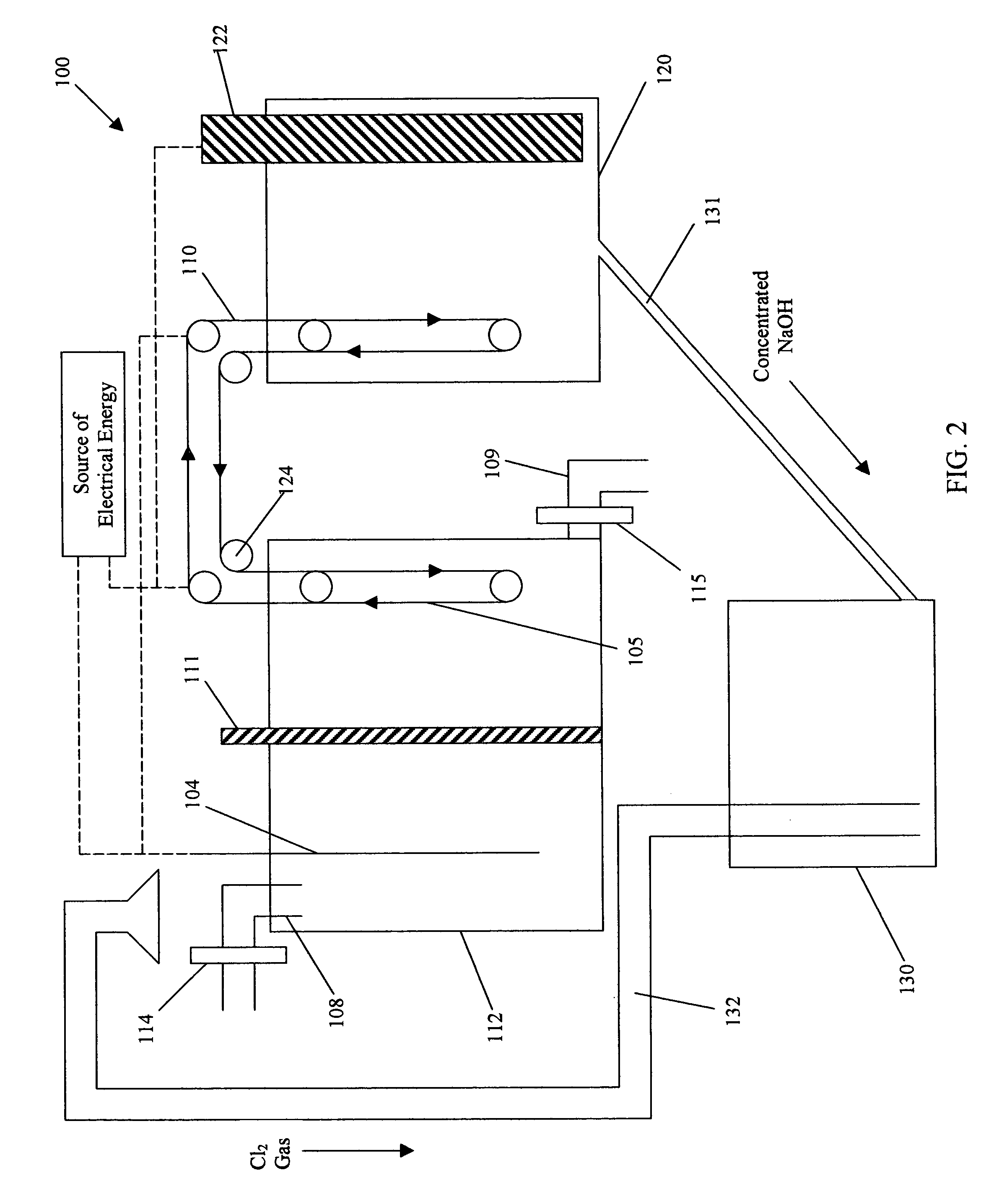
Process and apparatus for removing chloride and sodium ions from an aqueous sodium chloride solution
InactiveUS20050092618A1Reduce salt (NaCl) contentFuel and primary cellsCellsSodium amalgamTriple junction
The present invention discloses a process and apparatus for removing sodium and chloride ions from an aqueous sodium chloride solution, such as seawater or brine. The process includes electrolyzing aqueous sodium chloride to remove chloride and sodium ions in the form of chlorine gas and sodium metal. Preferably, a photovoltaic device, such as a triple junction amorphous silicon solar cell, provides the electrical energy for the electrolysis. The process utilizes electrode material that facilitates the production of chlorine gas and inhibits the evolution of hydrogen from the aqueous sodium chloride solution. The sodium is deposited onto a metal surface having a high hydrogen overpotential to produce sodium amalgam. The processed solution from the electrolysis has a reduced sodium chloride content and may be further processed to produce fresh water for human consumption or agricultural purposes. The sodium amalgam is removed from the aqueous sodium chloride solution and transported to and coupled against an air depolarizing fuel cell in water to produce electrical power with the sodium air fuel cell, power that may be used to operate the apparatus or other machinery. The product of the reaction between the sodium amalgam and the fuel cell is sodium hydroxide that may be reacted with the chlorine gas to produce sodium hypochlorite.
Owner:ENERGY CONVERSION DEVICES INC
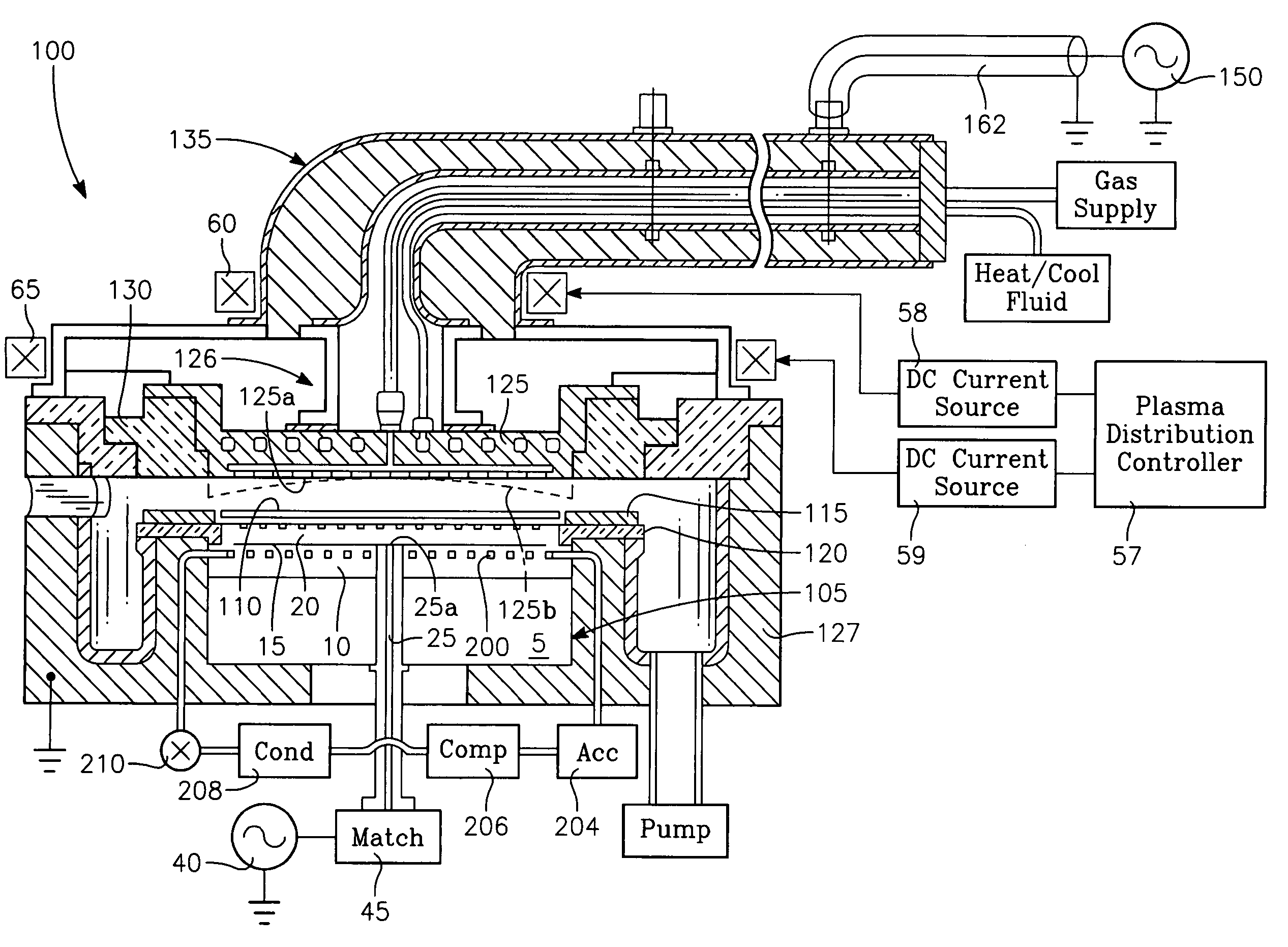
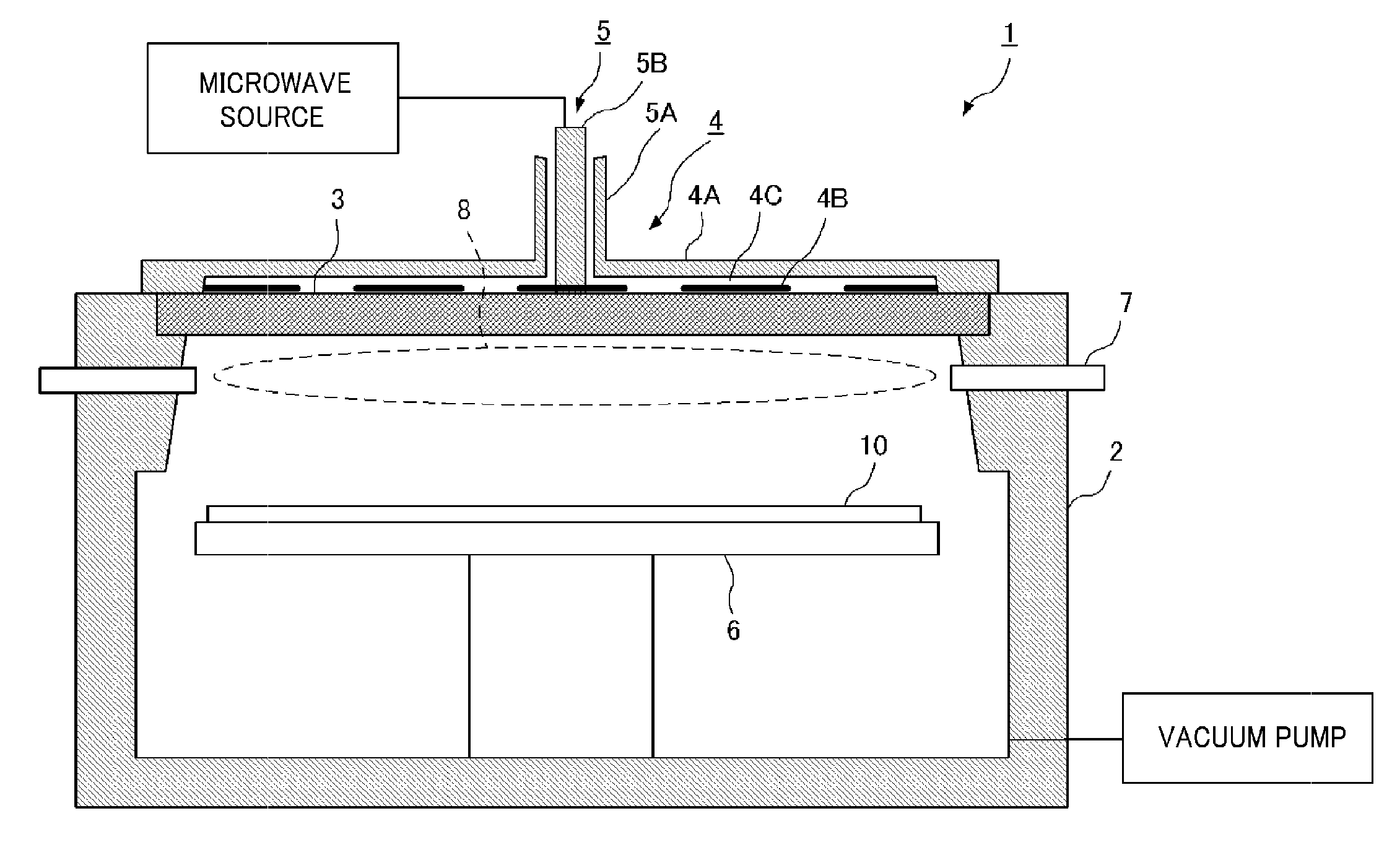
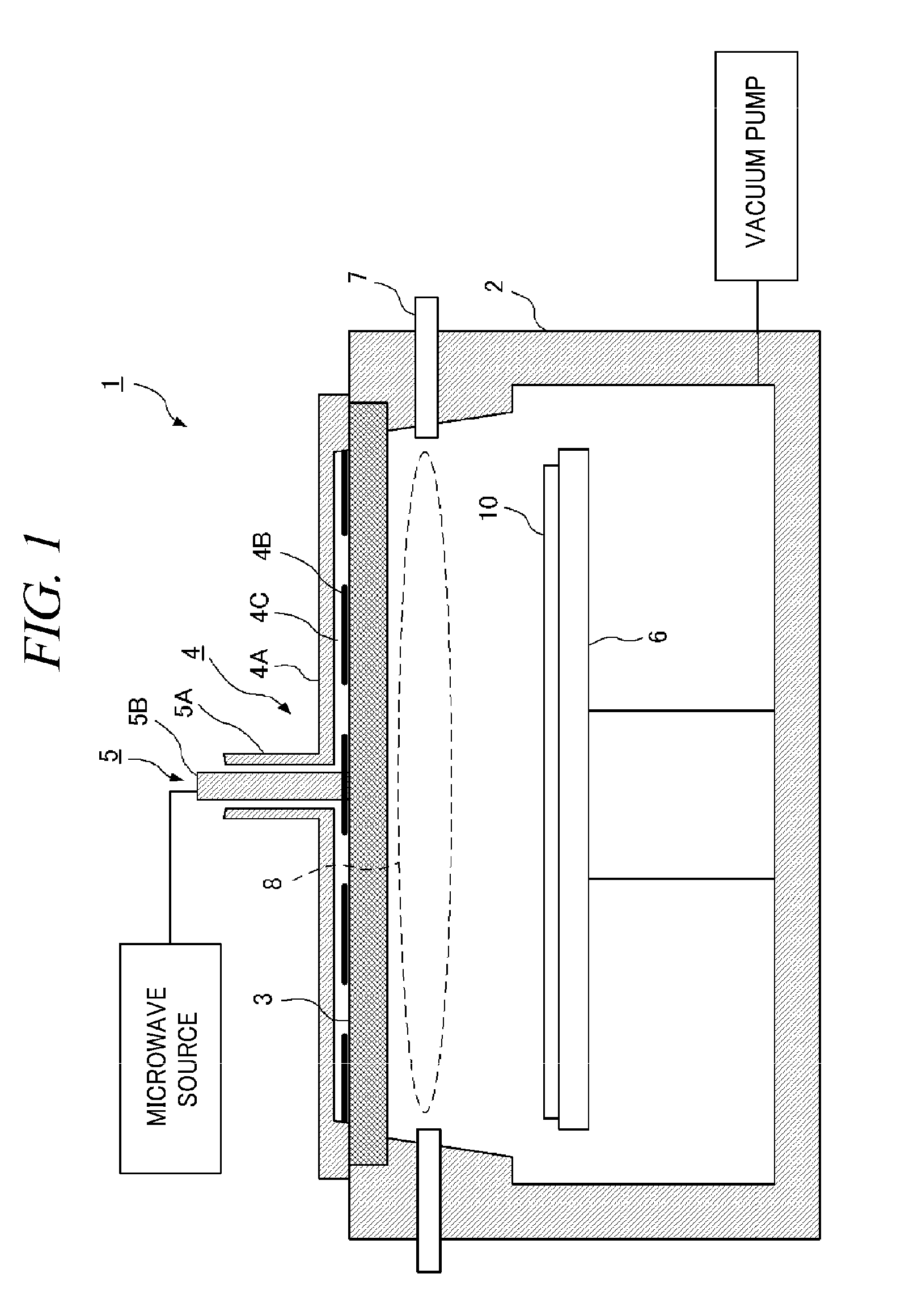
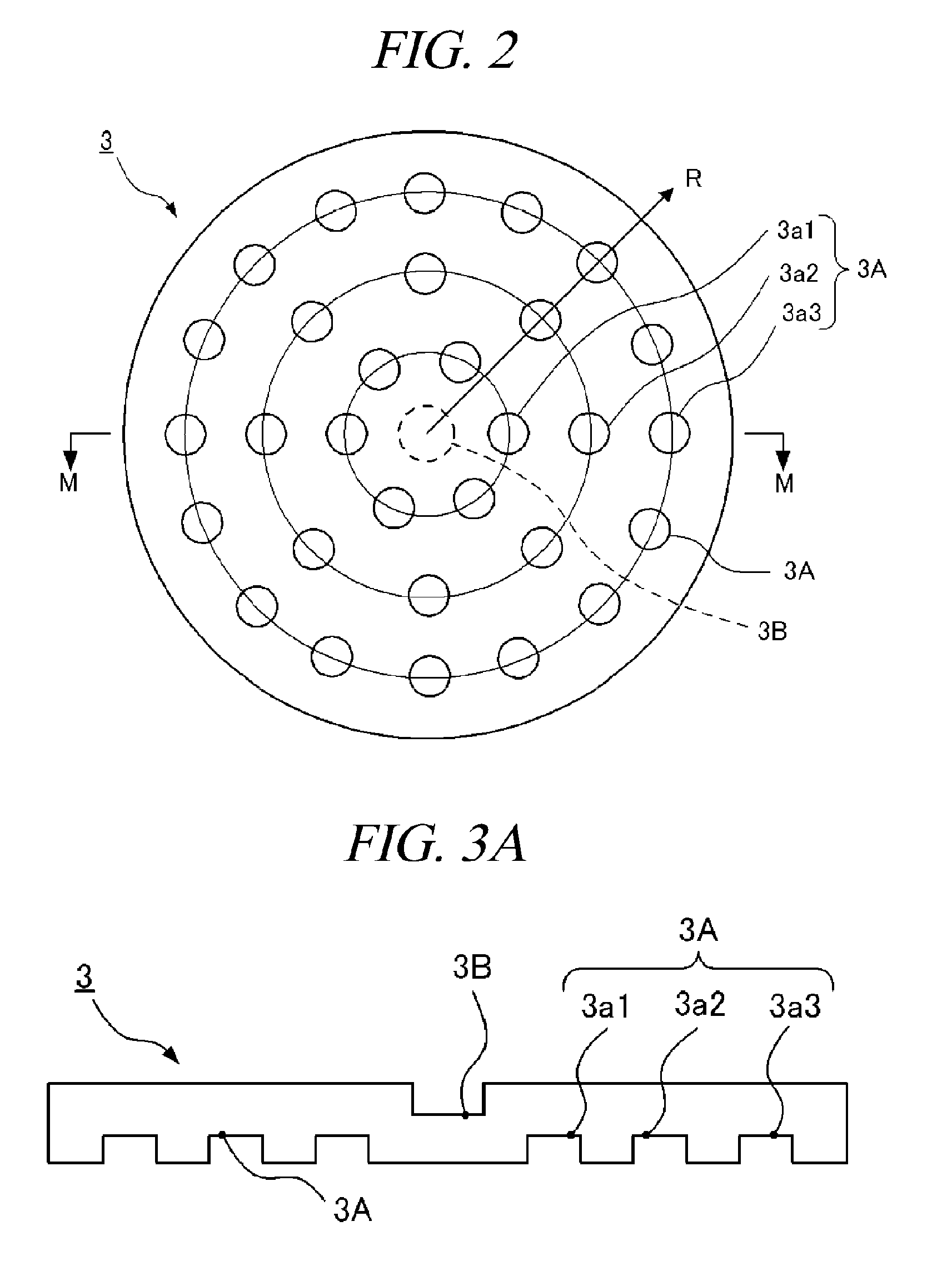
Top plate of microwave plasma processing apparatus, plasma processing apparatus and plasma processing method
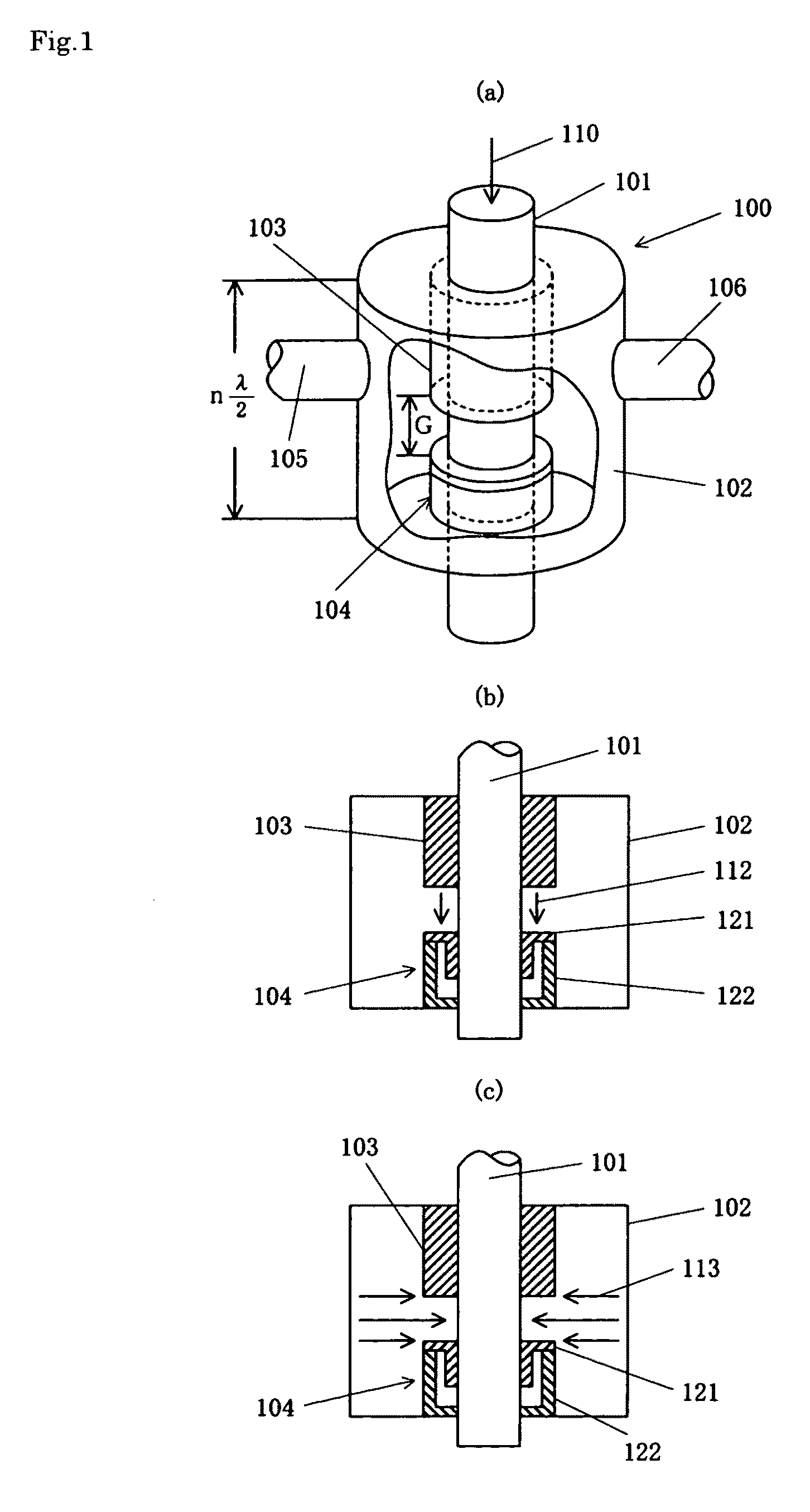
ActiveUS20110000780A1Uniform and stable plasmaIon sources/gunsElectrogenerative processesResonanceMicrowave electromagnetic radiation
A plasma generation chamber of a plasma processing apparatus is closed by a top plate 3. The top plate 3 has recesses 3A on its surface facing the plasma generation chamber and a central recess 3B on an opposite surface. The top plate 3 is coupled to an antenna thereon. If a microwave is supplied to the antenna, the microwave is radiated through slots of the antenna. The microwave is propagated through the top plate 3 such that the microwave has a plane of polarization and the microwave forms a circularly polarized wave as a whole. Here, resonance absorption of the microwave occurs at a side surface of recesses 3A and the microwave is propagated within the recesses 3A in a single mode. Strong plasma can be generated within each of the recesses 3A, so that a stable plasma mode can be generated in the top plate 3.
Owner:TOKYO ELECTRON LTD
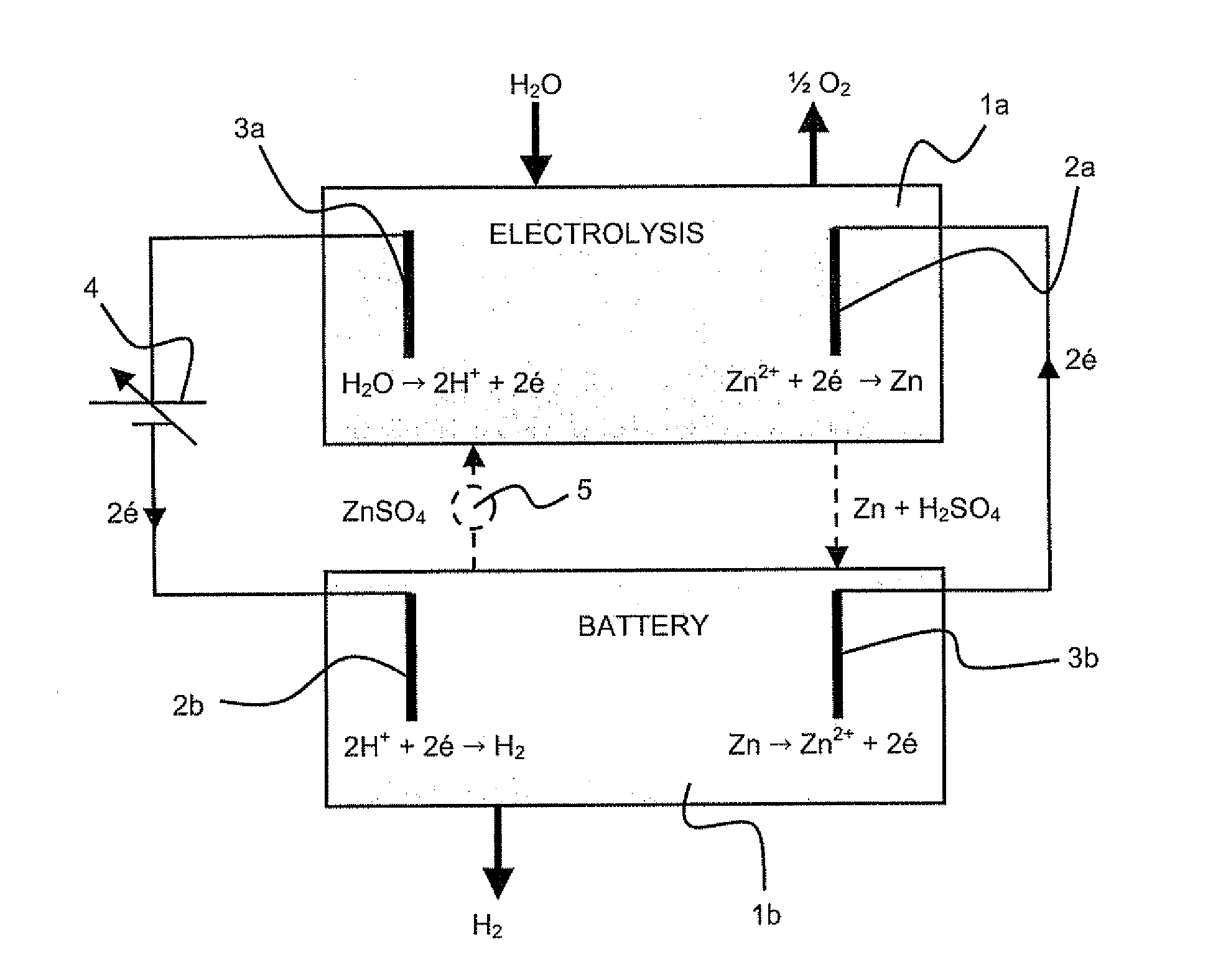
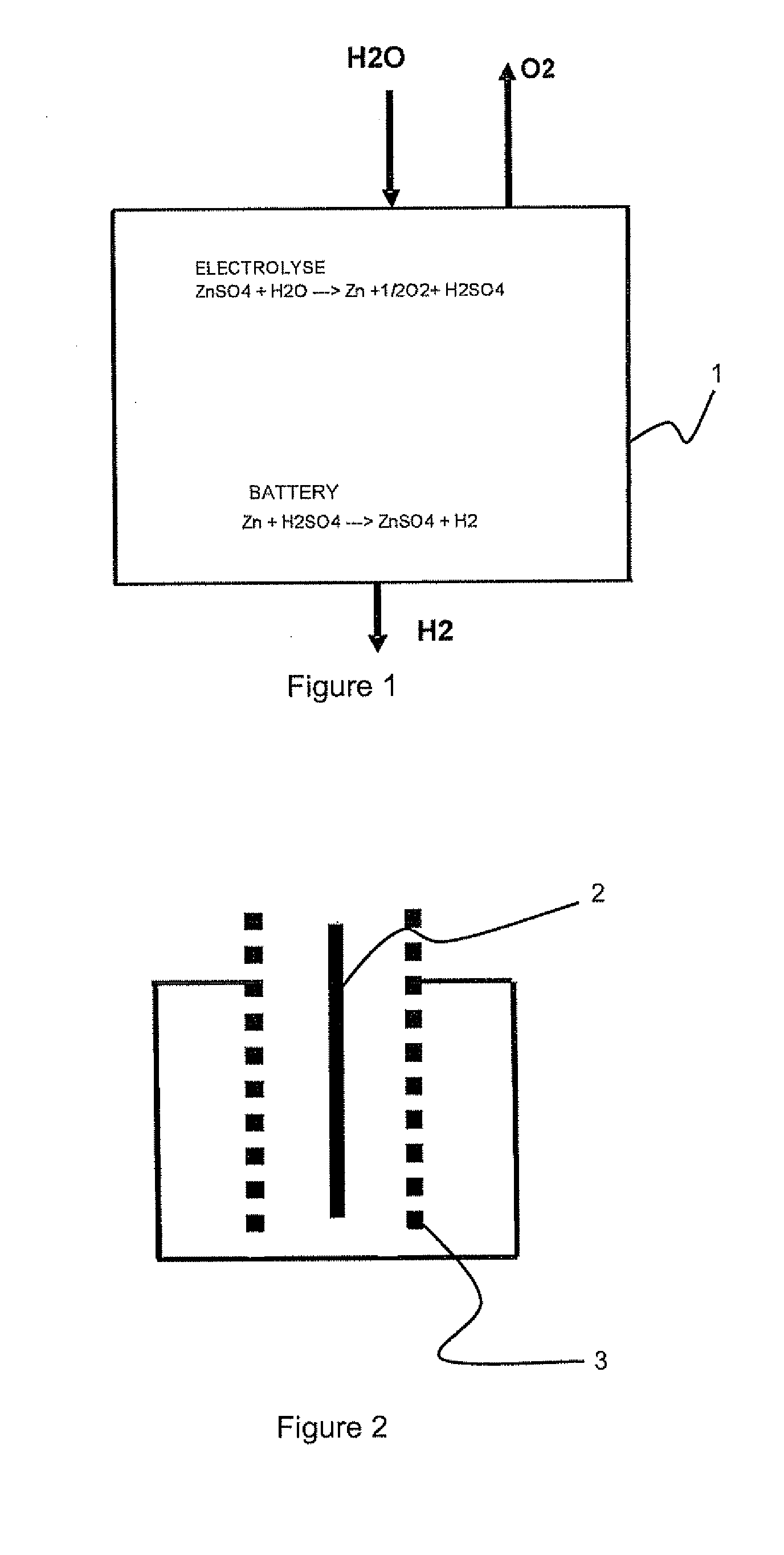
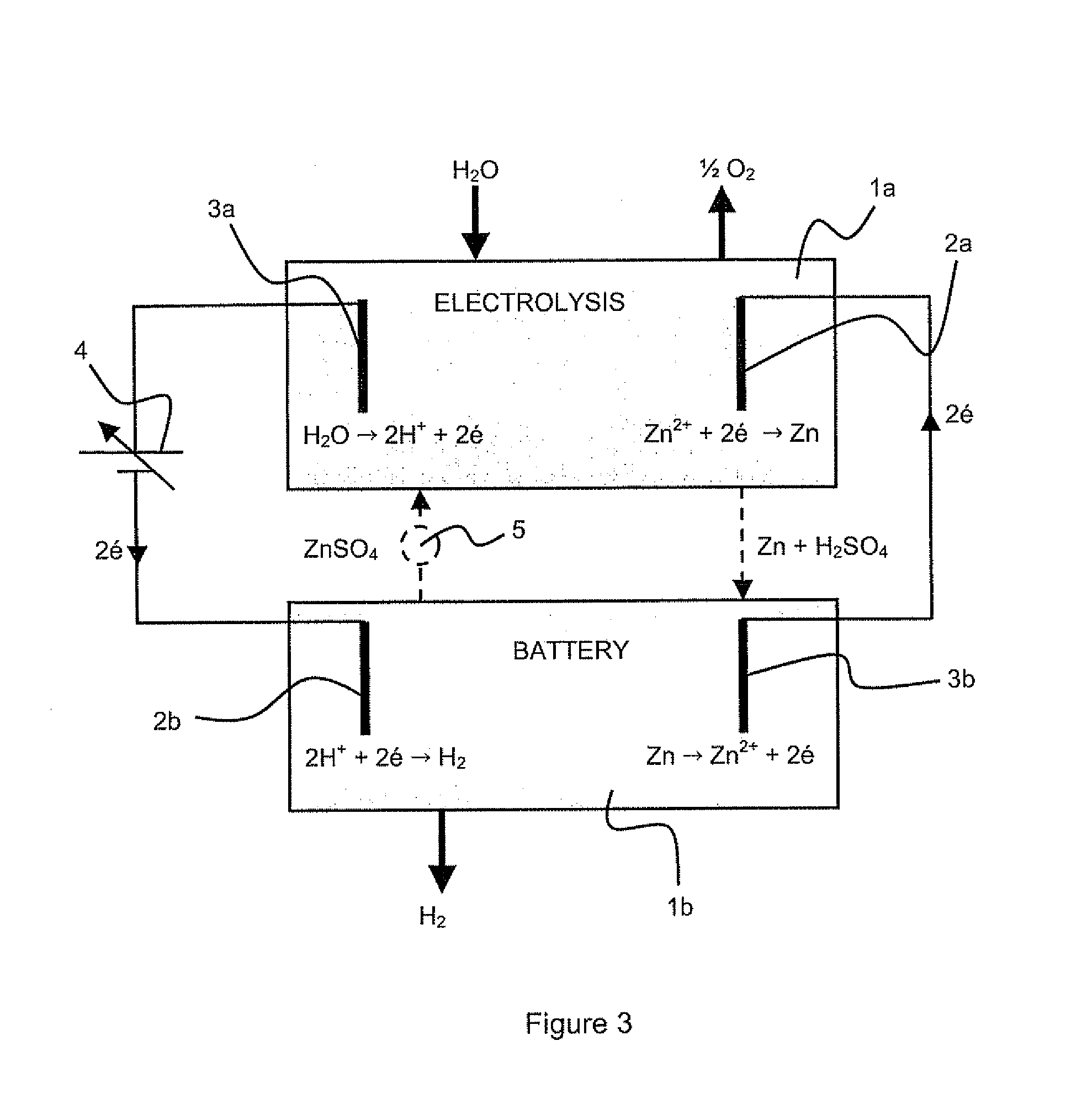
Method for co-generation of electric energy and hydrogen
ActiveUS20120121998A1Provides flexibility of usingPromote productionPhotography auxillary processesElectrolysis componentsElectricityElectrolysis
A method for simultaneous co-generation of electric energy and hydrogen by totally electrochemical means which includes an electricity storage phase by electrolysis of an electrolysable metal solution and formation of a hydrogen-electrolysable metal battery cell and, an electricity recovery and hydrogen generation phase by operation of said battery cell. The electrolysable metal is chosen from zinc, nickel and manganese.
Owner:ERGOSUP
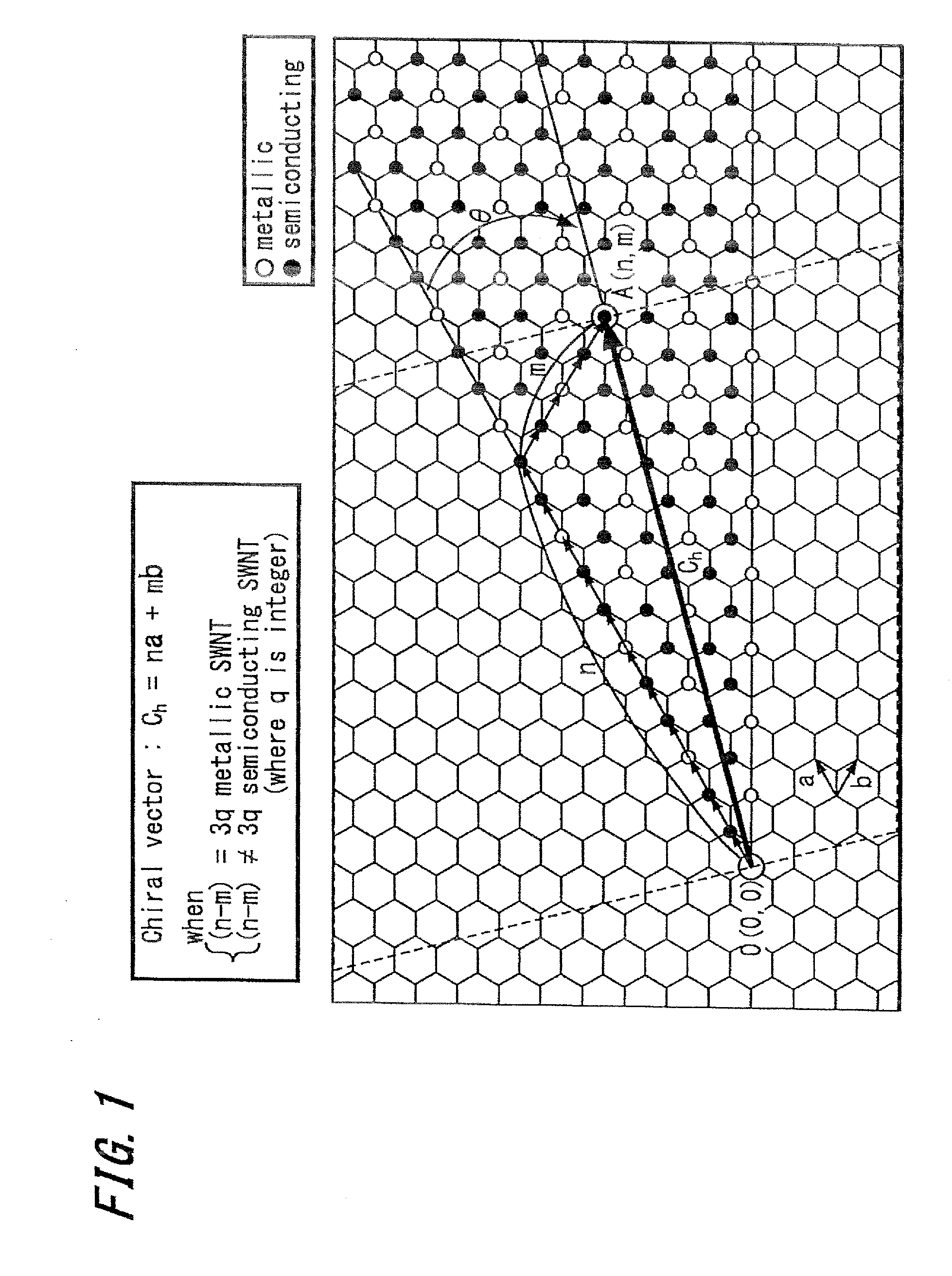
Carbon nanotube composition, method for manufacturing the same, array, and electronic device
InactiveUS20090253590A1High selectivityMaterial nanotechnologyElectrolysis componentsCarbon nanotubeChirality
The present invention attempts to establish a method for surface-fixing single-walled carbon nanotubes having a desired chirality highly selected from among the single-walled carbon nanotubes having various chiralities, and utilizes the method to provide an array of the carbon nanotubes for electronic devices. The present invention attempts also to provide a carbon nanotube composition including carbon nanotubes having a single chiral vector (n, m) at a purity of more than 50% based on the unit of number wherein n and m are integers, and a method for manufacturing the same.
Owner:HOKKAIDO UNIVERSITY
Electrocatalysts and additives for the oxidation of solid fuels
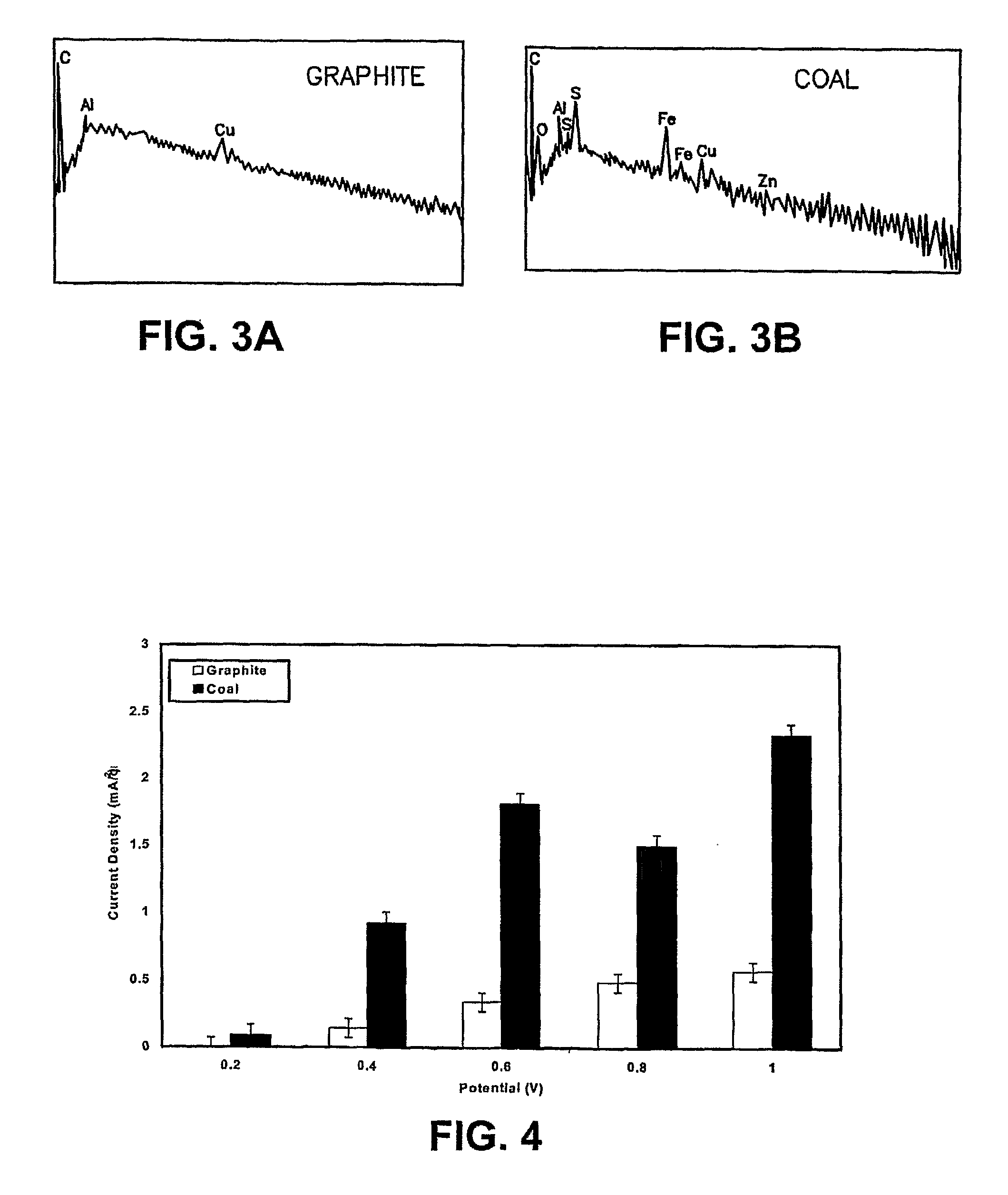
A continuous coal electrolytic cell for the production of pure hydrogen without the need of separated purification units Electrodes comprising electrocatalysts comprising noble metals electrodeposited on carbon substrates are also provided. Also provided are methods of using the electrocatalysts provided herein for the electrolysis of coal in acidic medium, as well as electrolytic cells for the production of hydrogen from coal slurries in acidic media employing the electrodes described herein. Further provided are catalytic additives for the electro-oxidation of coal. Additionally provided is an electrochemical treatment process where iron-contaminated effluents are purified in the presence of coal slurries using the developed catalyst.
Owner:OHIO UNIV
Method for reclamation of precious metals from circuit board scrap
InactiveUS6986192B2Small footprintInexpensiveElectrostatic separationLayered productsDenatured alcoholHigh rate
The present invention springs from the discovery that mild acids could be utilized to shear undesired metals away from desired precious metals that have been plated onto circuit board runners or contacts. This shearing action occurs at a high rate when metal scrap segments are immersed in mild acid and excited by application of an electromagnetic field at specific frequencies and power levels. These frequencies and power levels are based on the end metal desired and the metals contained in the scrap and the acid utilized. When mild acid saturated with copper sulfate and loaded with scrap metals is subjected to an electromagnetic field at the appropriate frequency and power levels copper and nickel molecules are sheared rapidly and absorbed into solution, leaving only the desired metal, such as gold, in a 99.5% pure flake which can be skimmed off the surface of the solution or filtered from the solution. The captured metal flake is then rinsed in water and denatured alcohol, compressed, melted and poured into bars or nuggets for further use or sale.
Owner:FITCH MICHAEL K
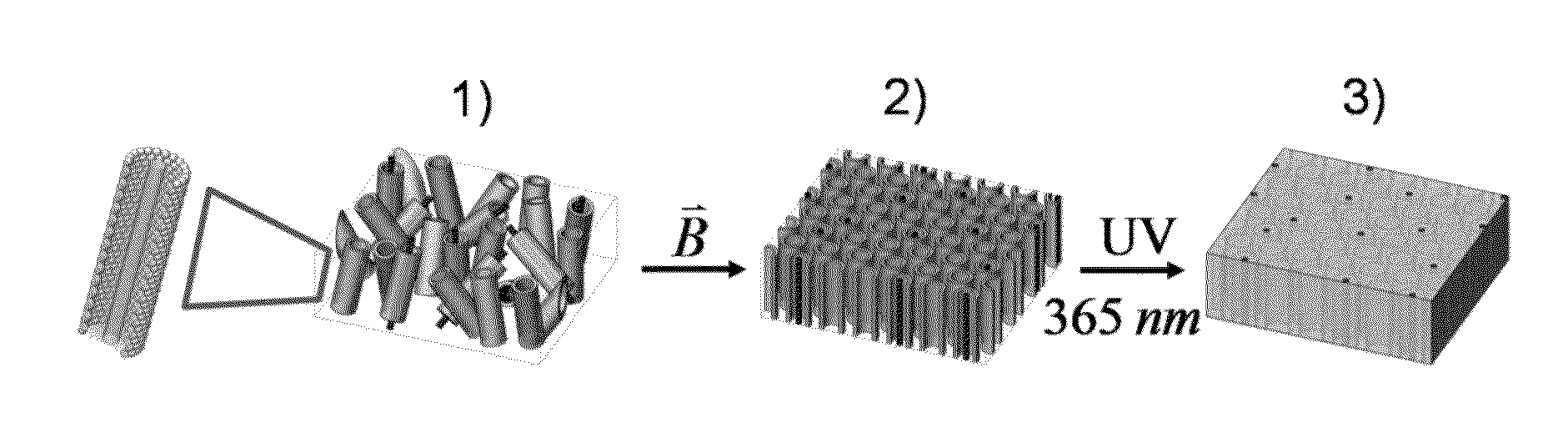
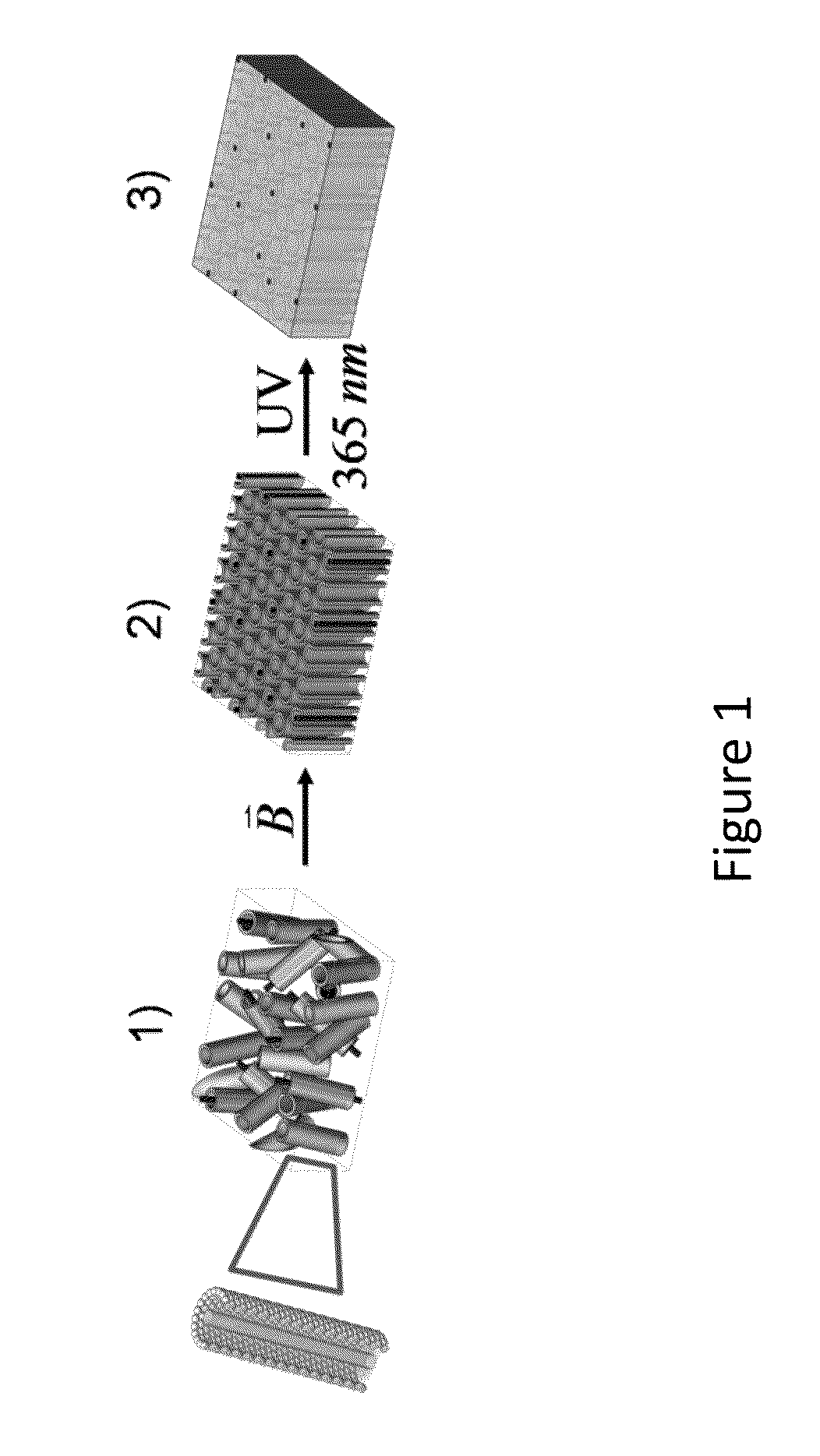
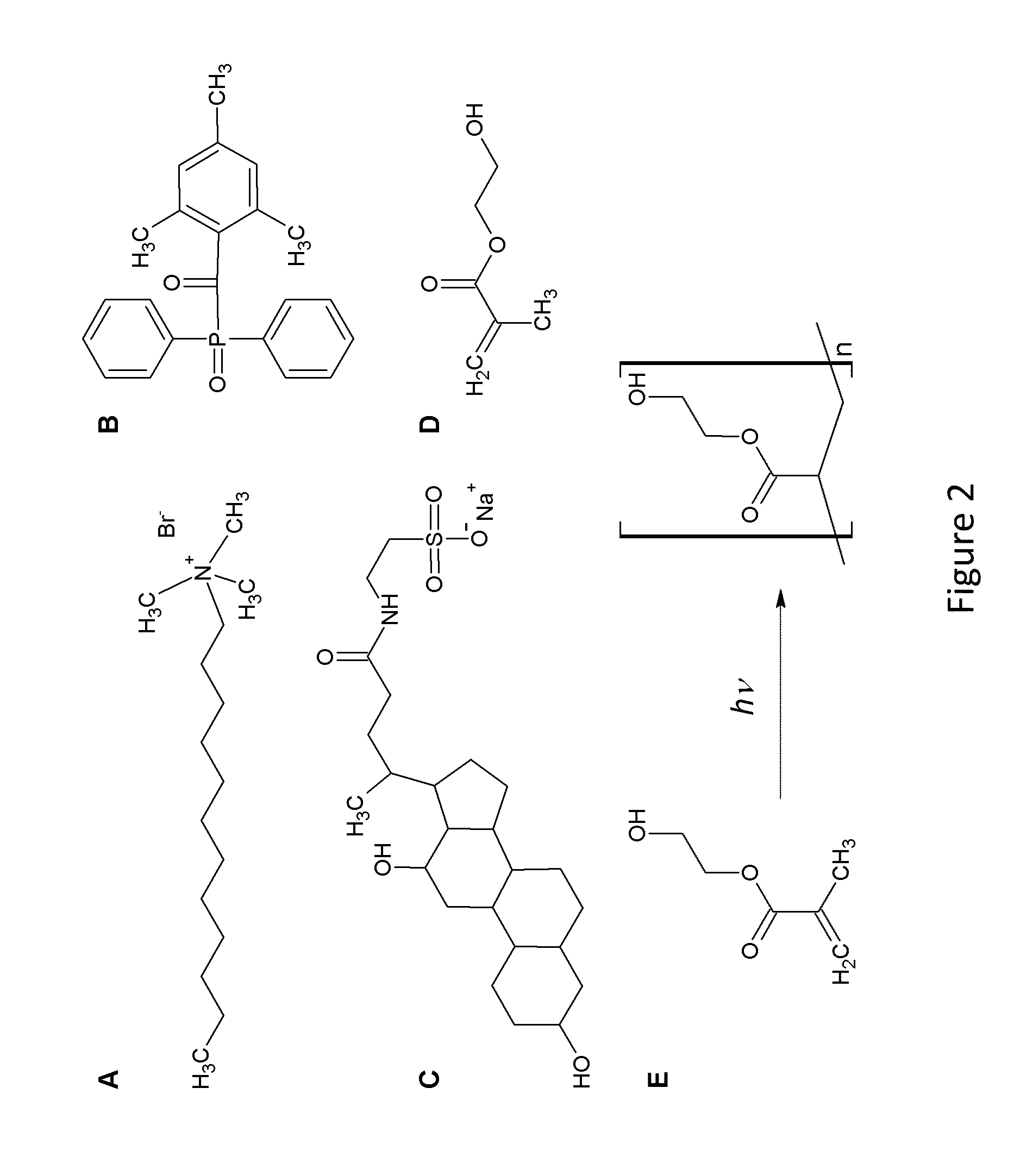
Polymeric Composites Having Oriented Nanomaterials and Methods of Making the Same
ActiveUS20130090405A1Material nanotechnologyElectrogenerative processesPolymer compositesPolymer nanocomposite
The present invention relates to the development and fabrication of thin-film polymer nanocomposites containing vertically aligned nanomaterials, such as single-walled carbon nanotubes (SWNTs). In certain embodiments, the present invention utilizes liquid crystal mesophases of hexagonally packed cylindrical micelles that orient with their long axes parallel to an applied magnetic field, thereby directing the alignment of the nanomaterials, such as SWNTs, sequestered in the micellar cores. In certain embodiments, the mesophase may be a stable, single-phase material containing monomers that can be polymerized after nanotube alignment to form the nanocomposite polymer.
Owner:YALE UNIV
Method for Producing Nano-Scale Low-Dimensional Quantum Structure, and Method for Producing Integrated Circuit Using the Method for Producing the Structure
InactiveUS20070287202A1Increase temperatureImprove availabilityMaterial nanotechnologyCarbon compoundsCarbon nanotubeCarbon source
A method of an embodiment of the present of the present application is for producing a nano-scale low dimensional quantum structure. The method includes: bringing a catalyst on a substrate into contact with vaporized carbon source, and emitting an electromagnetic wave to the catalyst so as to form single-walled carbon nano-tubes on the catalyst. As a result, it is possible to form the nano-scale low-dimensional quantum structure on a target area.
Owner:JAPAN SCI & TECH CORP
Reactor, process, and system for the oxidation of gaseous streams
InactiveUS20140275679A1Electrolysis componentsElectrolytic organic productionElectrical conductorLiquid fuel
A reactor and process capable of concurrently producing electric power and selectively oxidizing gaseous components in a feed stream, such as hydrocarbons to unsaturated products, which are useful intermediates in the production of liquid fuels. The reactor includes an oxidation membrane, a reduction membrane, an electron barrier, and a conductor. The oxidation membrane and reduction membrane include an MIEC oxide. The electron barrier, located between the oxidation membrane and the reduction membrane, is configured to allow transmission of oxygen anions from the reduction membrane to the oxidation membrane and resist transmission of electrons from the oxidation membrane to the reduction membrane. The conductor conducts electrons from the oxidation membrane to the reduction membrane.
Owner:BIO2ELECTRIC
Plasma generator and method of generating plasma using the same
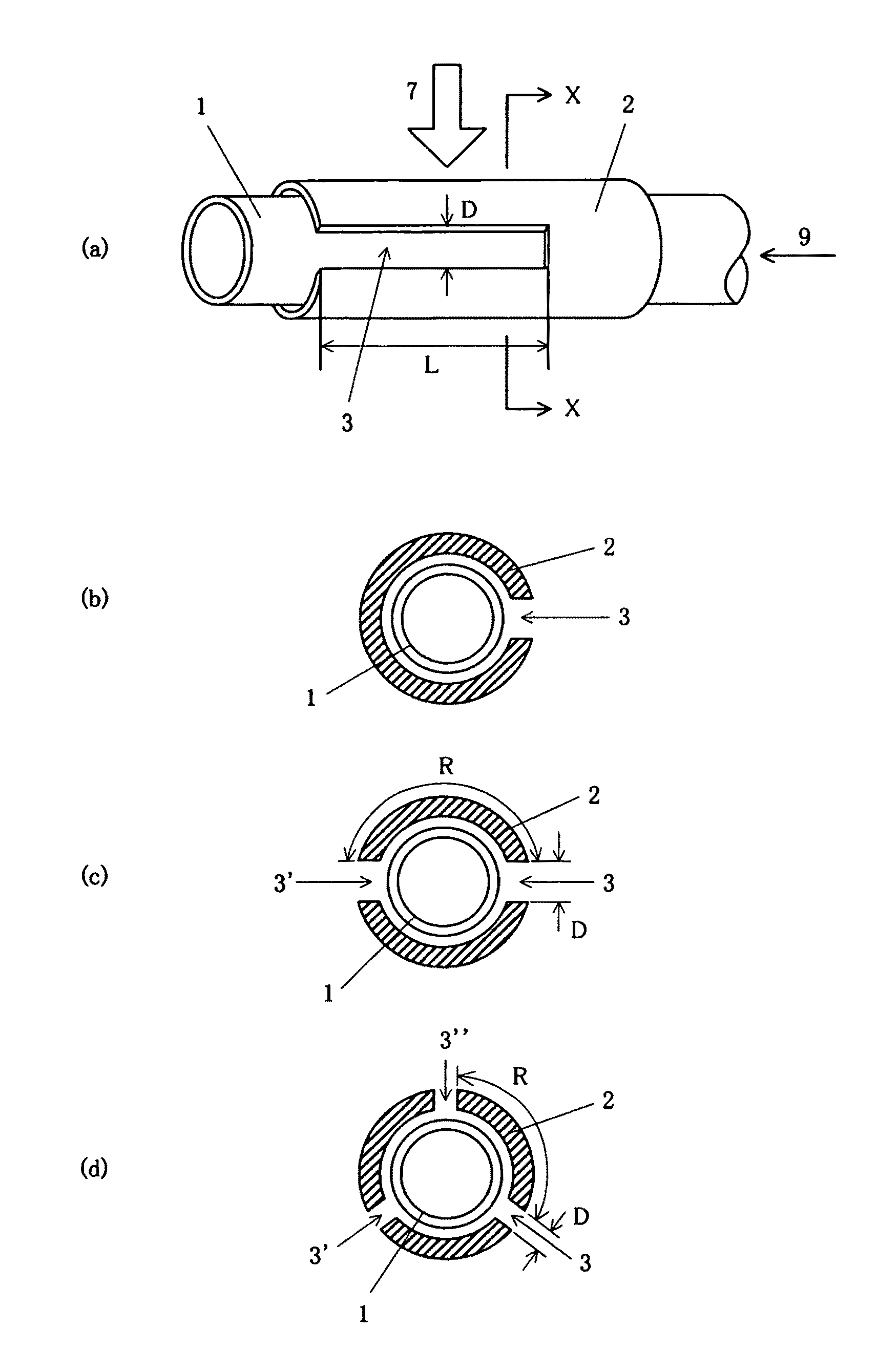
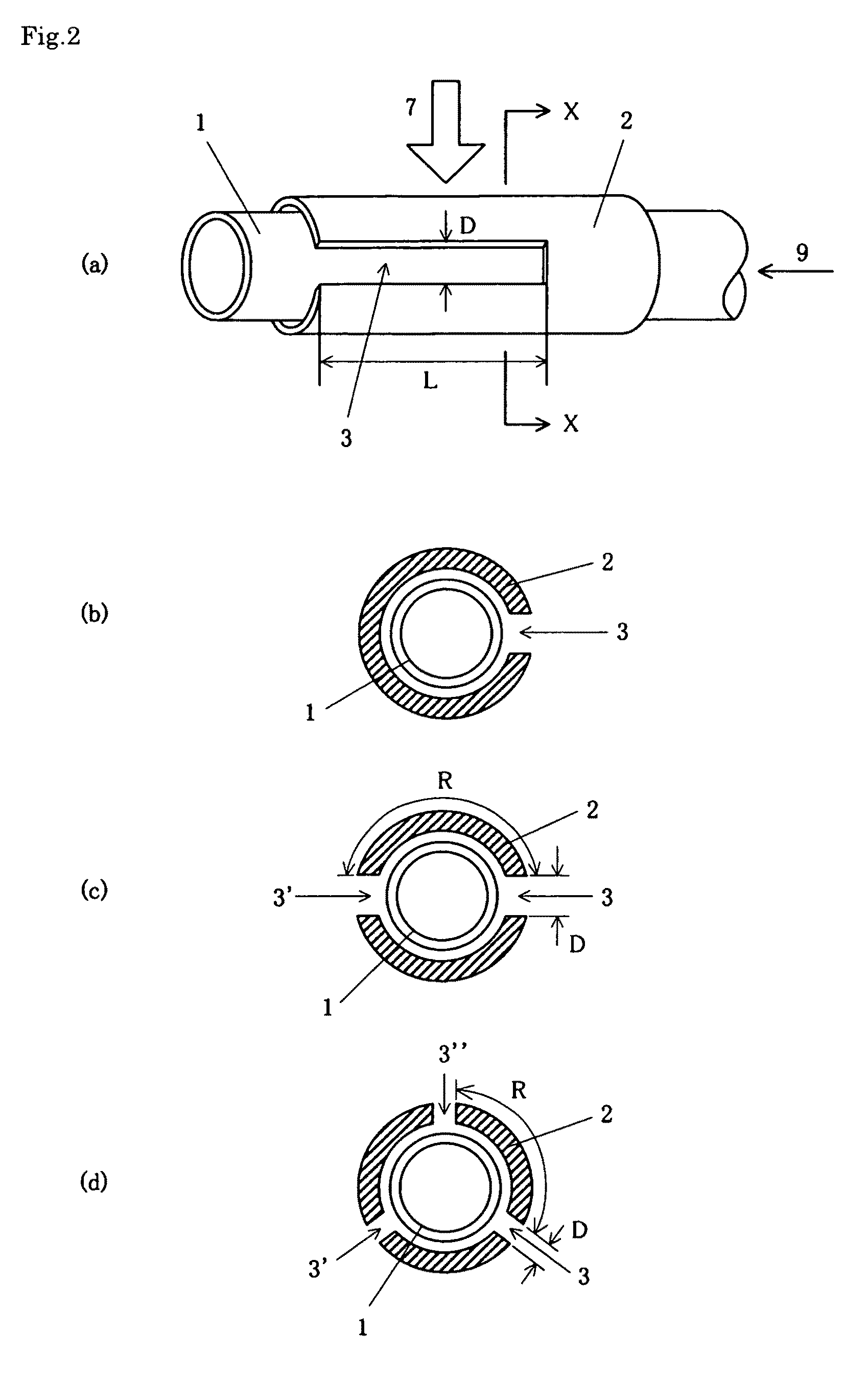
InactiveUS8216433B2Avoid changes in impedanceWeaken energyElectric discharge tubesGas-gas reaction processesMicrowavePlasma generator
A plasma generator in which the variation of the impedance in the cavity before and after plasma is ignited is less and hardly affected by the shape of the cavity, and the ignitability of the plasma is improved and a method of generating plasma using the plasma generator are provided. The plasma generator comprises a nonconductive gas flow pipe (1) for introducing a gas (9) for generating plasma and discharging it into the atmosphere and a conductive antenna pipe (2) surrounding the gas flow pipe. A microwave (7) is applied to the antenna pipe to change the gas in the gas flow pipe into plasma. The plasma generator is characterized in that a slit (3) with a predetermined length is formed in the antenna pipe (2) along the axial direction of the gas flow pipe. Preferably, the plasma generator is characterized in that the length of the slit is an integral multiple of the half-wave length of the applied microwave.
Owner:UNIVERSITY OF THE RYUKYUS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com