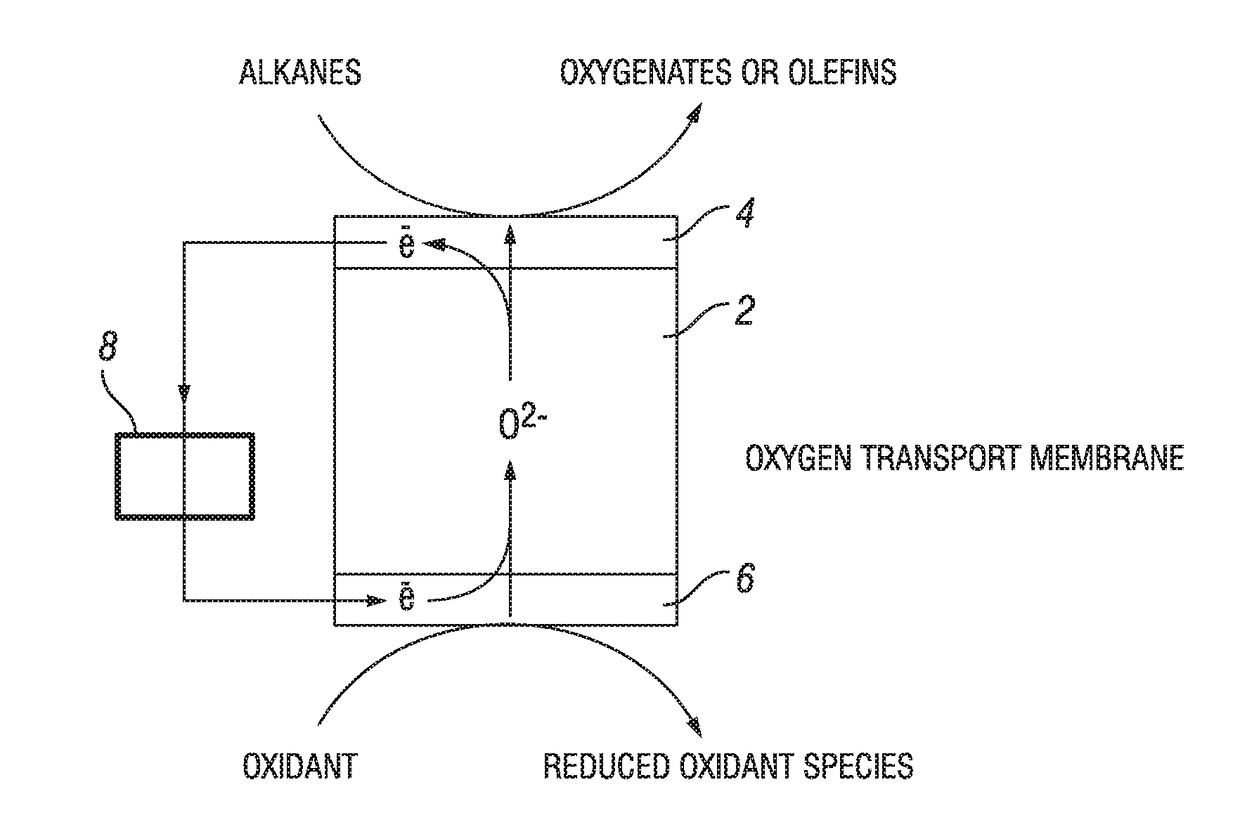
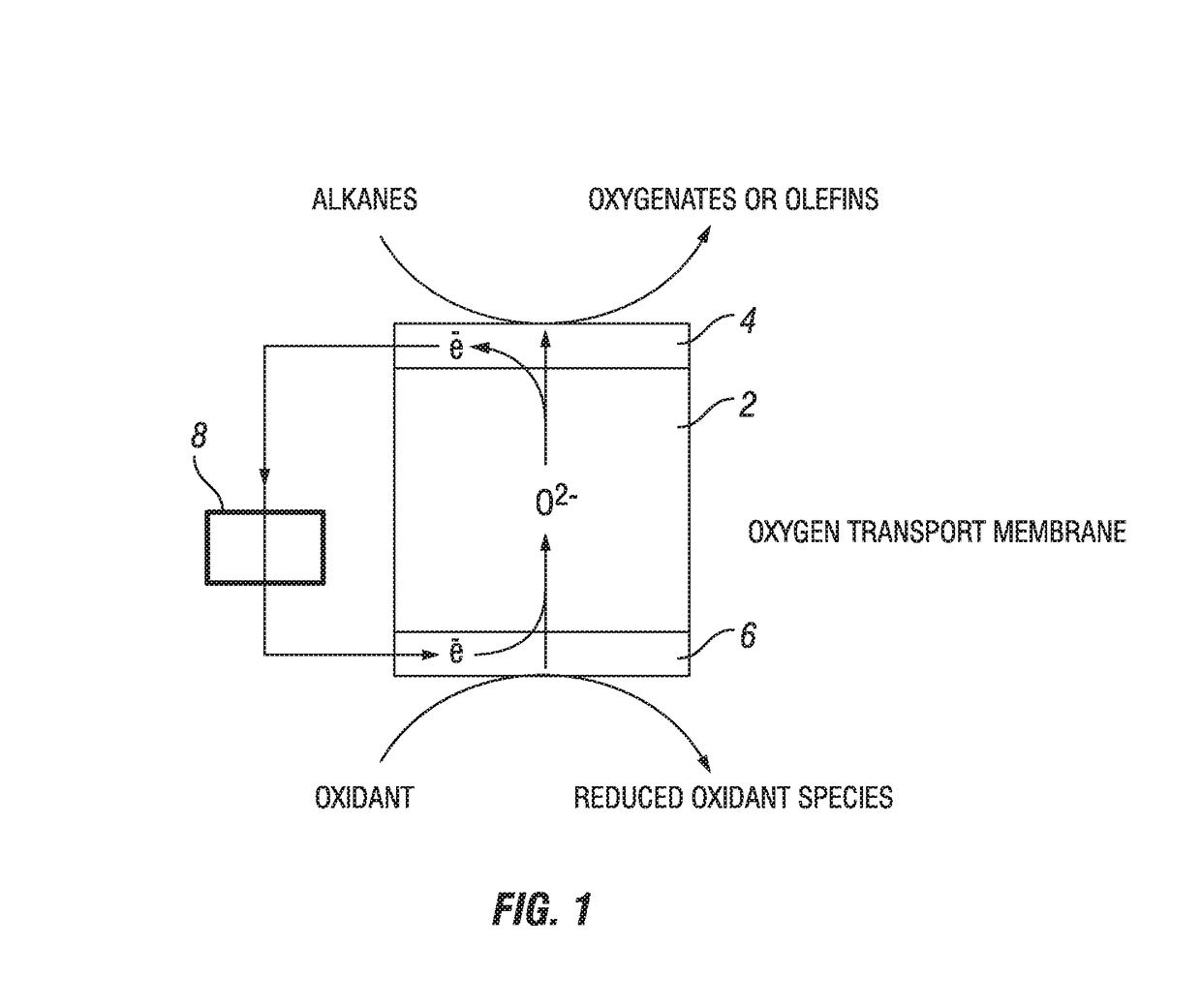
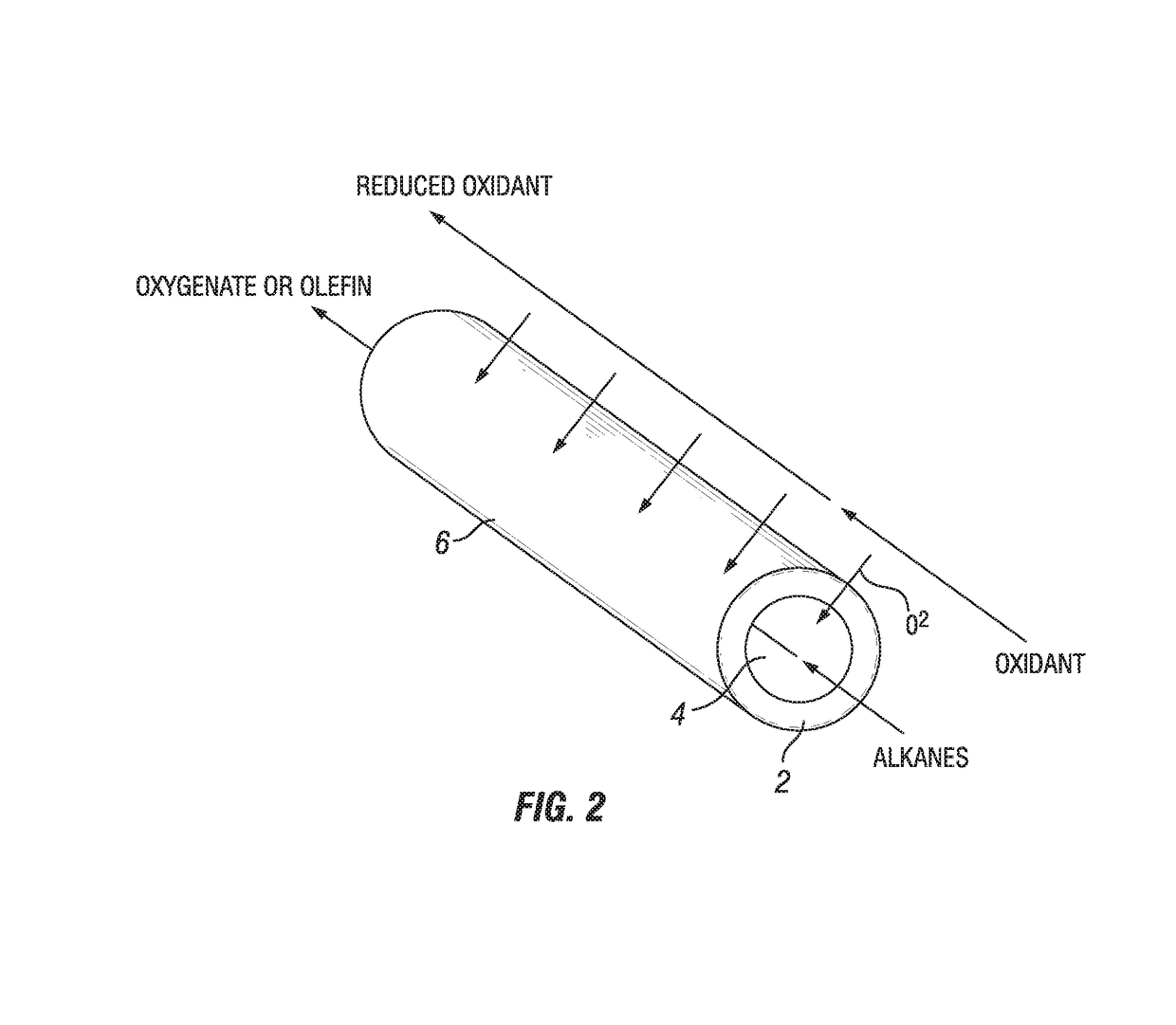
Process for oxidation reactions
a technology of oxidation reaction and process, which is applied in the direction of electrolytic organic production, multiple component coatings, electrolytic coatings, etc., can solve the problems of difficult one-pot direct conversion of paraffins to oxygenates or olefins, inability to activate methane and reaction with oxygen to directly produce methanol in a commercial-scale continuous process, and low single-pass conversion of methane to methanol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0042][NH4][ZSM-5] (SiO2 / Al2O3=50) catalyst was crushed to a size of 20-40 mesh. The catalyst was calcined in a muffle furnace at 550° C. to convert the ammonium form to the acidic form, H-ZSM-5. Samples were heated to 150° C. at 2° C. / min, maintained at 150° C. for 2 hours, heated to 450° C. at 4° C. / min, maintained at 450° C. for 12 hours, then heated to 550° C. at 4° C. / min and held 550° C. for 6 hours. Following calcination, the catalyst was cooled to ambient temperature.
[0043]The resulting H-ZSM-5 powder was sieved to a particle size of <140 mesh and suspended in terpineol such that the resulting suspension was 35% H-ZSM-5 by weight. The H-ZSM-5 / terpineol suspension was then mixed with an equal weight of lanthanum strontium manganite (LSM) electrode ink.
[0044]The experiment used an electrolyte-supported button cell with total active electrode area of 0.71 cm2. The electrolyte material was scandia-stabilized zirconia (ScSZ). The cathode side of the electrolyte was coated with LS...
example 2
[0053]Strontium chloride (267 mg), lanthanum nitrate (1300 mg), and manganese sulfate (845 mg) were dissolved in distilled water (2 mL). This solution was added dropwise to ZSM-5 (4 g) with mechanical stir bar mixing. This wet powder was calcined in air to generate the oxide (500 C, 30 minutes).
[0054]One gram of the resulting dark powder oxide was combined with one gram of terpineol. This slurry was combined with 2 grams of commercially available lanthanum strontium manganite (LSM). A portion of this mixture was then deposited uniformly in a 1 cm diameter circle onto a 1 inch diameter electrolyte disc. This deposition was carried out by screen printing. The cell was heated to 125° C. for 30 min to remove the terpineol solvent.
[0055]An identical screen-printed deposition, this time of the pure commercial LSM, was carried out on the opposite face of the electrolyte disc, overlapping as exactly as possible with the deposition on the original side, sandwiching the electrolyte disc in be...
PUM
| Property | Measurement | Unit |
|---|---|---|
| operating temperature | aaaaa | aaaaa |
| α-oxygen capacity | aaaaa | aaaaa |
| electrical | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com