Solid multi-component membranes, electrochemical reactor components, electrochemical reactors and use of membranes, reactor components, and reactor for oxidation reactions
a multi-component membrane and reactor technology, applied in the field of electrochemical reactors, can solve the problems of poor product selectivity at high ethane conversion, adversely affecting product selectivity, and conventional oxydehydrogenation processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation examples
MULTI-COMPONENT MEMBRANE PREPARATION EXAMPLES
[0285] The multi-component membranes used in Examples A-1 to A-14 were prepared as follows.
example a
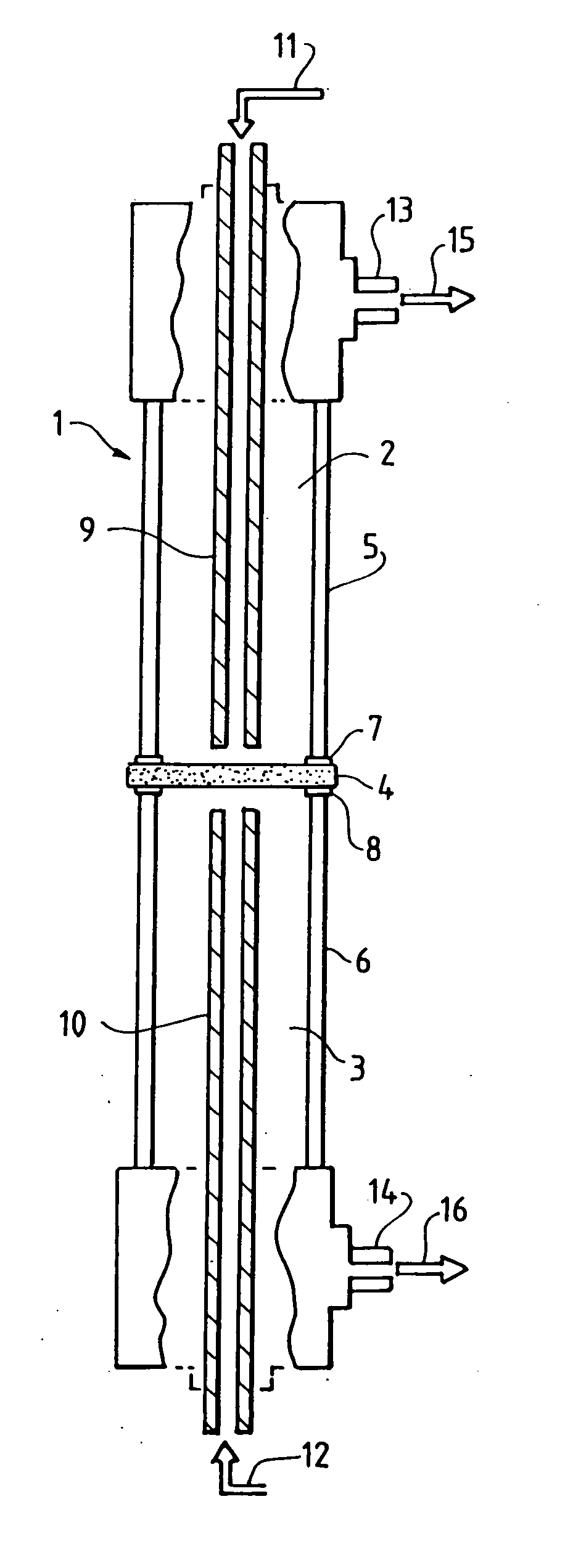
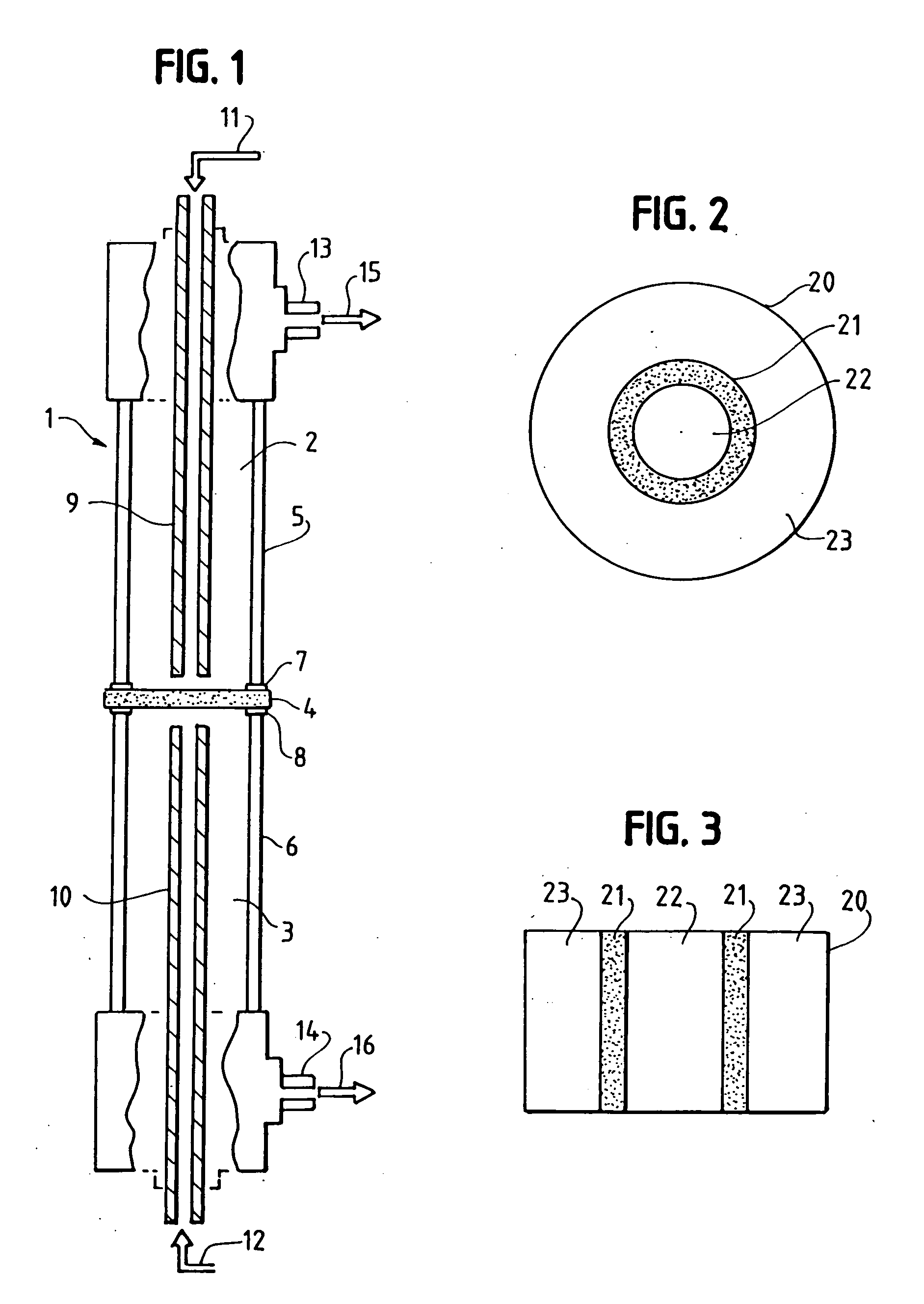
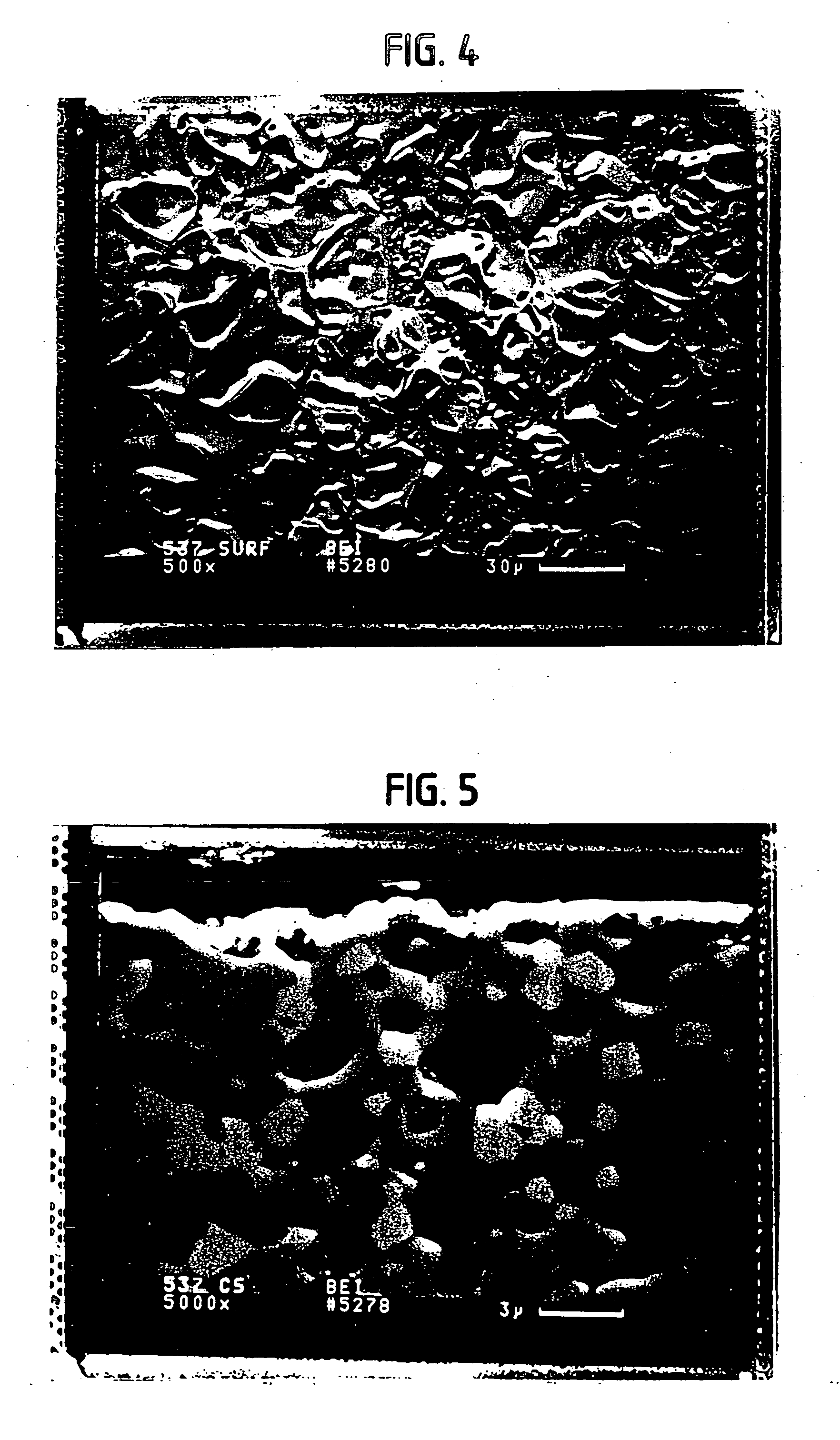
[0286] The dual conductor membrane used in Examples A-1 and A-2 below is fabricated by making a disk which contains palladium metal as the electronically-conductive phase and yttria-stabilized zirconia (hereinafter “YSZ”) as the ionically-conductive phase. A powder mixture of 50% each of palladium oxide and yttria (8 mol. %)-stabilized zirconia is first made. The powder is then heated in a mixture of hydrogen and nitrogen atmospheres at 400° C. for 15 minutes to reduce the palladium oxide to palladium metal. To 4.0 grams of the mixture are added 0.4 grams Carbowax 20M™ (obtained from Supelco) dissolved in chloroform, and the resulting mixture is dried at 85° C. The resulting Pd / yttria-stabilized zirconia / Carbowax 20M™ powder is pressed into a disk using 60,000 psi applied pressure. The disk is then sintered in air at 1500° C. for 30 minutes. The resultant disk is dense and gas tight. The disk is one inch in diameter and 0.03 inch (0.76 mm) thick.
example b
[0287] The dual-conductor membrane used in Example A-3 below is fabricated by making a disk which contains platinum metal as the electronically-conductive phase and YSZ as the ionically-conductive phase. 9.52 grams of Engelhard Platinum Ink (a product of Engelhard Corporation: Cat. no. 6926) is diluted with 3 cc of alpha-terpineol and then 2.00 grams of yttria (8 mol. %)-stabilized zirconia is admixed in the diluted ink. The mixture is evaporated to dryness and the terpineol burned off in an oven at 100° C. The dried mass is then pulverized and 8.49 grams of the dried, pulverized powder is added to 0.94 grams of Carbowax 20M™ dissolved in 20 cc of chloroform. The chloroform is evaporated off and the remaining powder is dried in an oven at 85° C. for about 30 minutes and the powder is lightly reground and seived through a 120 mesh seive. Then 5.00 grams of the seived Pt / yttria-stabilized zirconia / Carbowax 20M™ powder is pressed into a disk having a diameter of 1-⅜ inch (3.50 cm) usin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com