siRNA MICROBICIDES FOR PREVENTING AND TREATING DISEASES
a technology of microbicides and microorganisms, applied in the direction of drug compositions, organic chemistry, genetic material ingredients, etc., can solve the problems of significant drop in the hiv infection rate and the individual's higher risk of contracting hiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Methods
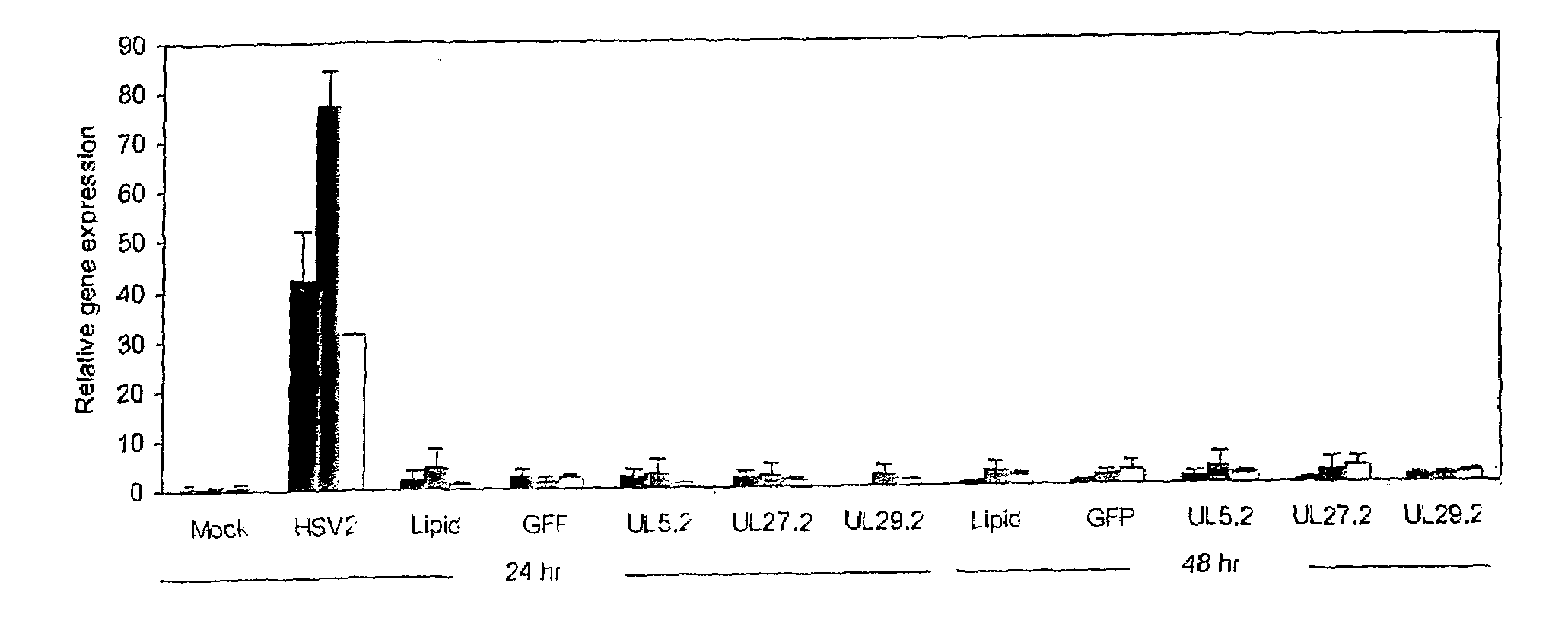
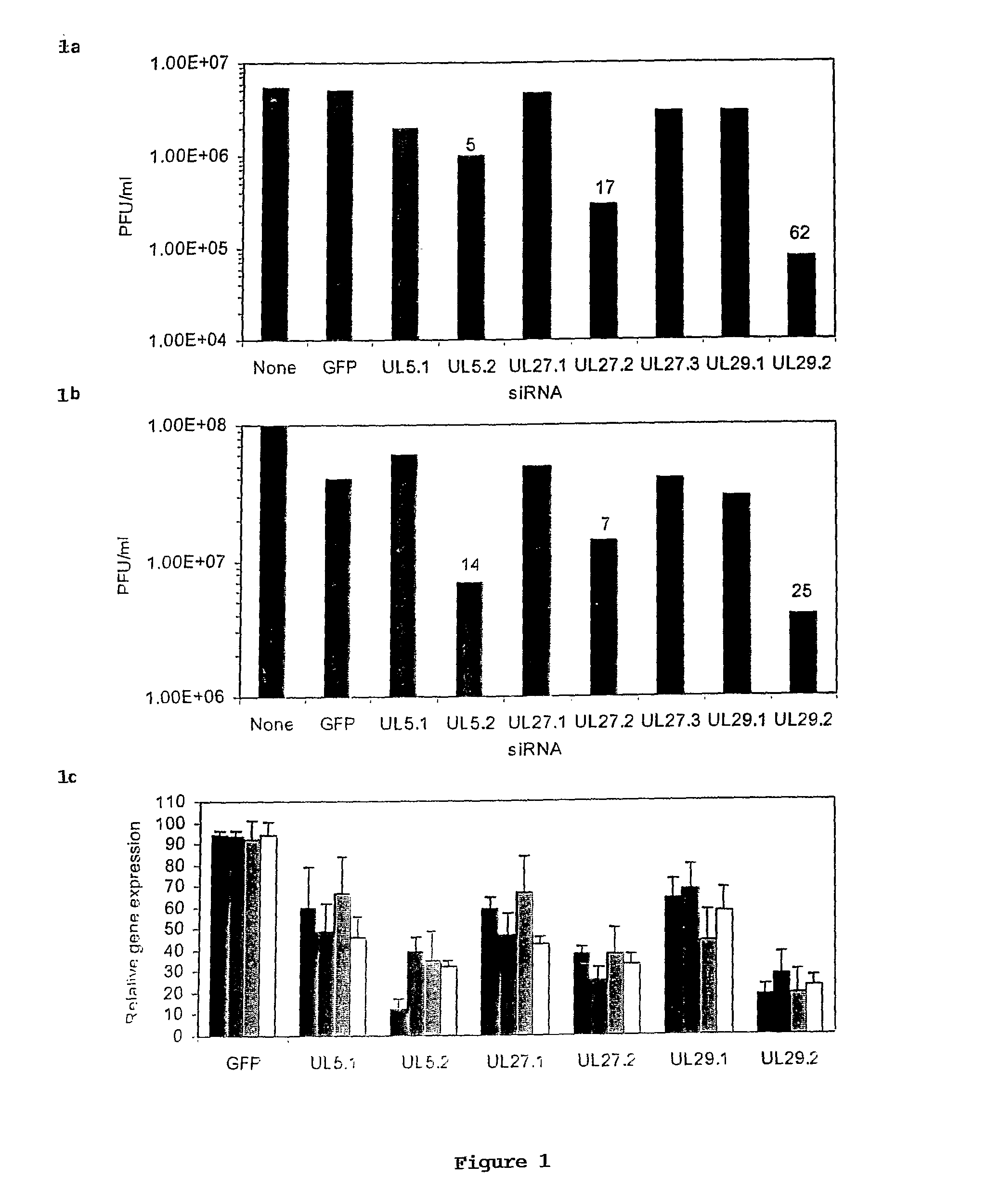
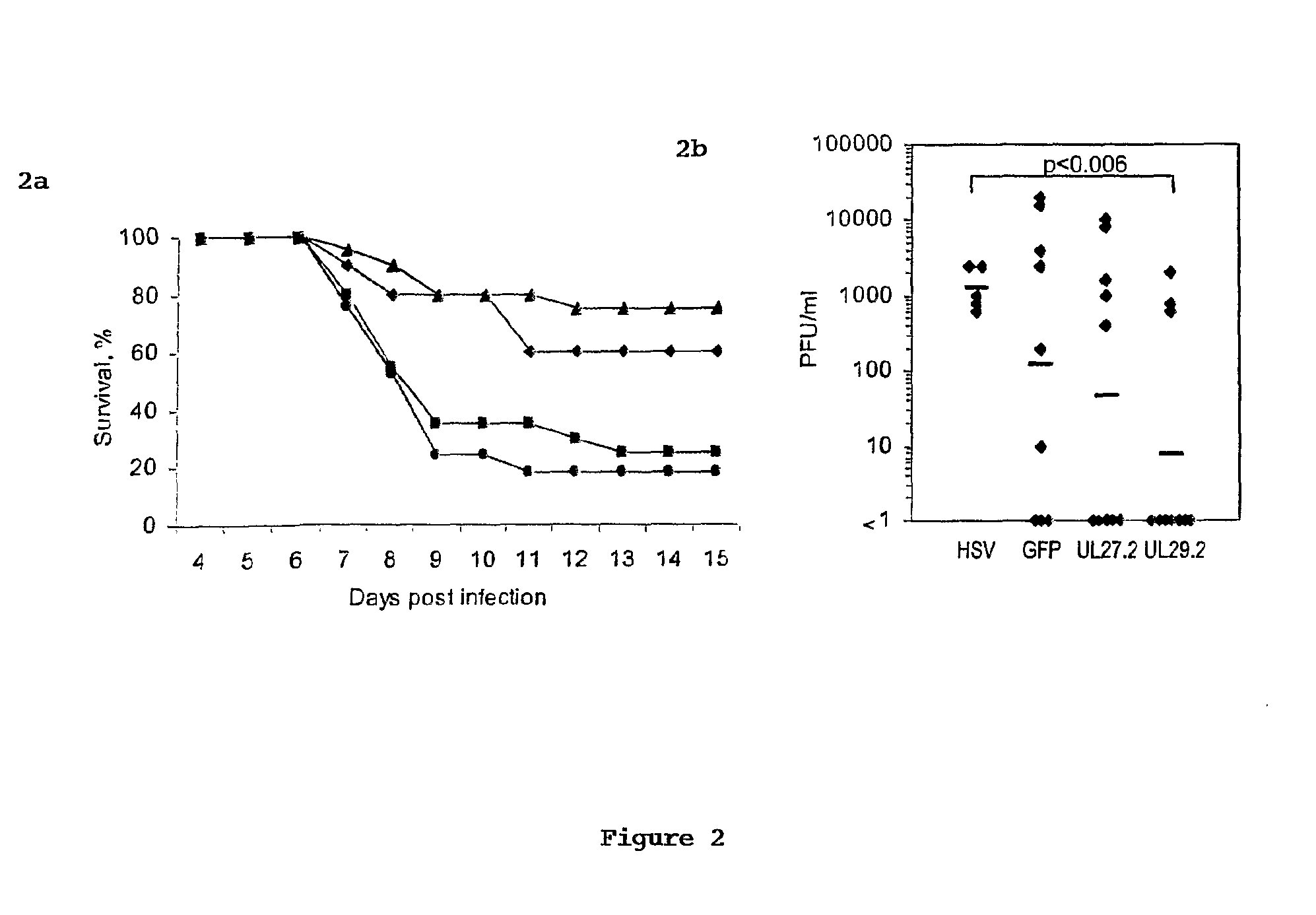
[0101]Mice. BALB / c mice (5-8 weeks old) were from Taconic Farms; FVB.Cg-Tg (GFPU)5Nagy mice were from Jackson Laboratories (11). Mice subcutaneously injected with 2 mg medroxyprogesterone acetate (Sicor) 1 wk earlier were infected with 104 (˜2 LD50) HSV-2 strain 186 per vagina (21). siRNA (500 pmole) complexed with Oligofectamine (Invitrogen), prepared according to the manufacturer's protocol, was administered per vagina in a maximal volume of 12 ul in two regimens—2 hr before and 4 hr after HSV-2 infection or 1 and 2 hr after HSV-2 infection. Mice were examined daily for signs of HSV-2 graded by a 5-point scale (0, no signs of infection; 1, slight genital erythema and edema; 2, moderate genital inflammation; 3, purulent genital lesions; 4, hind limb paralysis; 5, death) (21). Viral shedding from the genital epithelium was determined by swabbing (Micropur swab, PurFybr Inc) the vaginal cavity on day 6 post-infection and titrating virus on Vero cells. In some cases, the vagina...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Condensation enthalpy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com