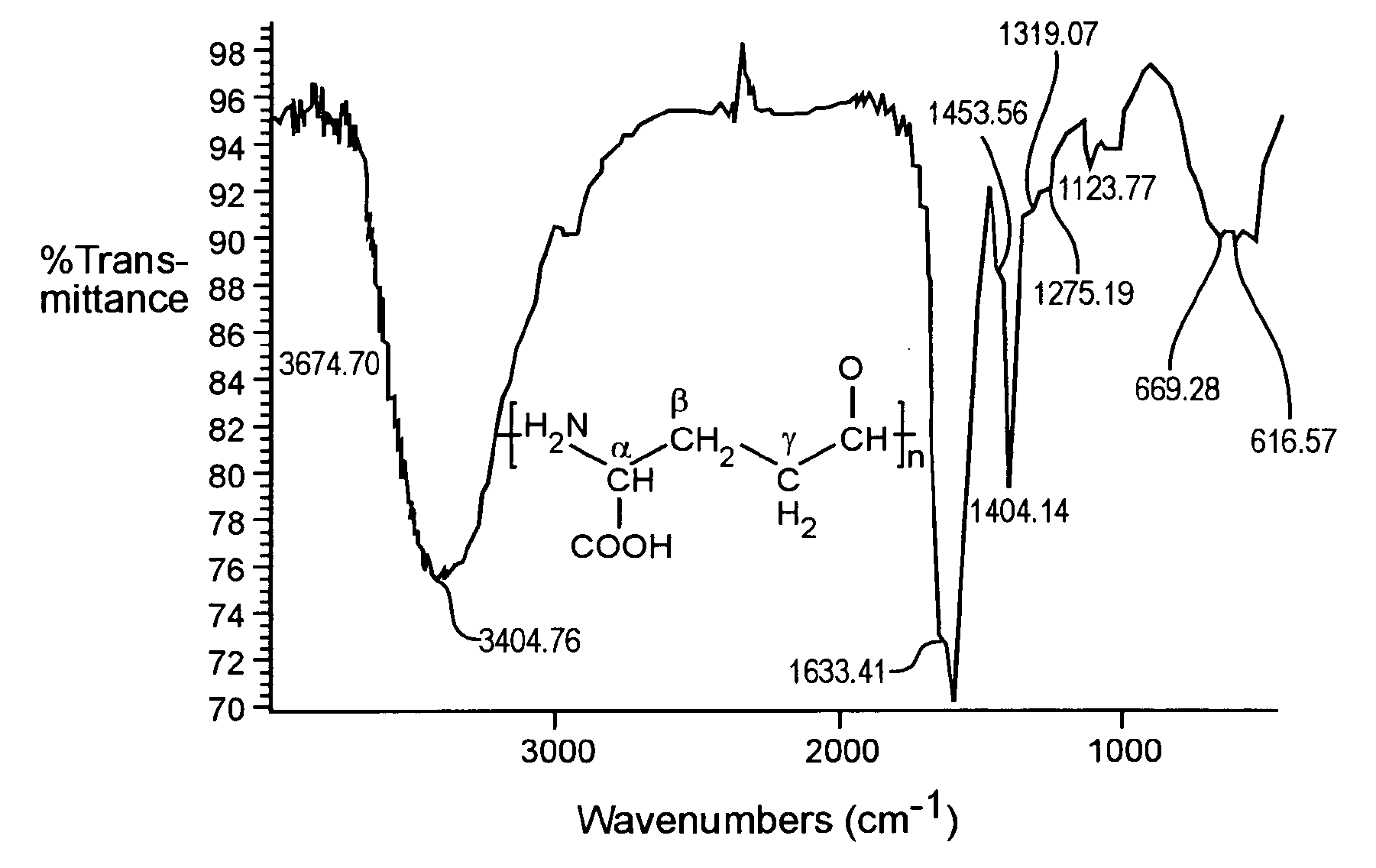
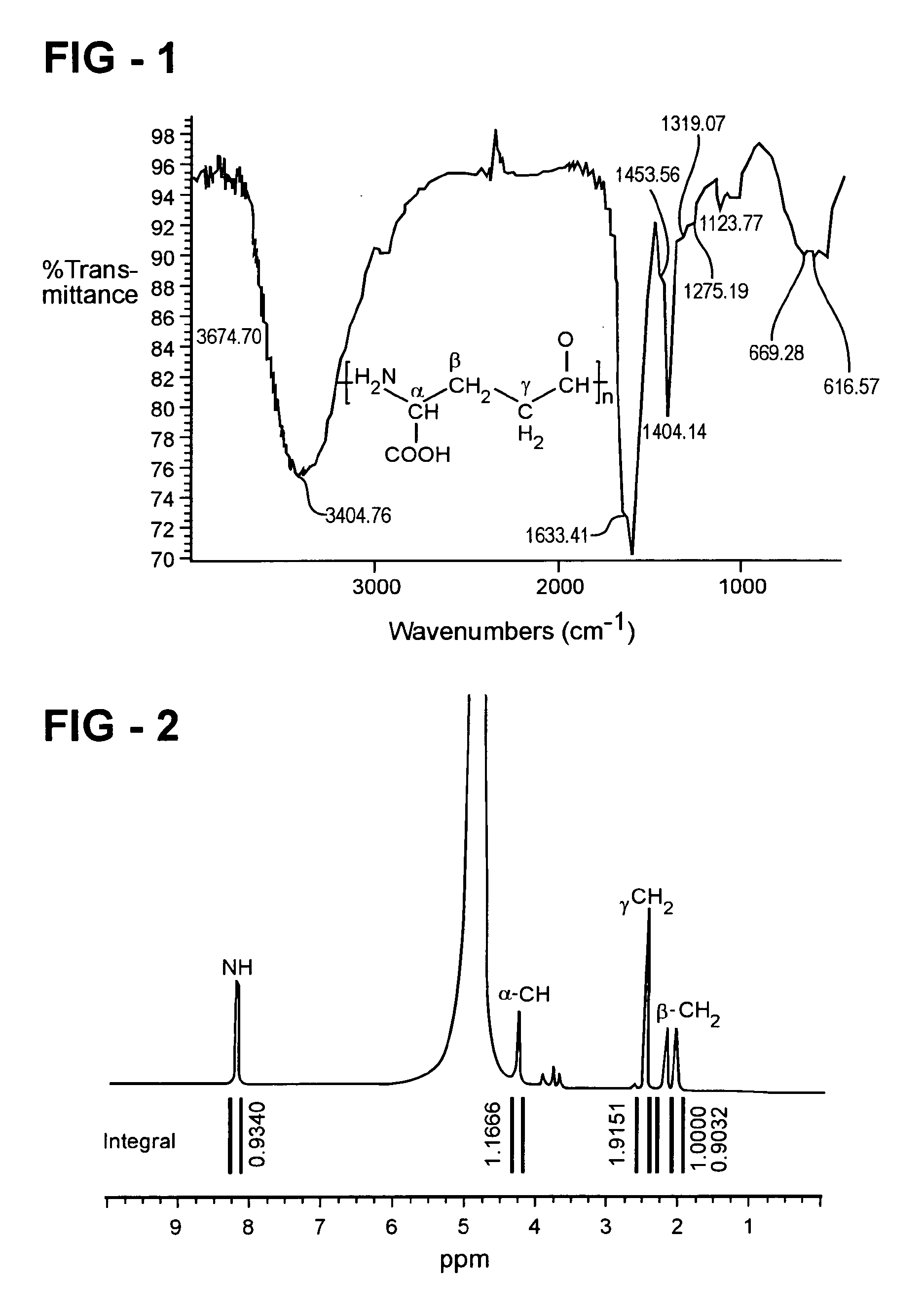
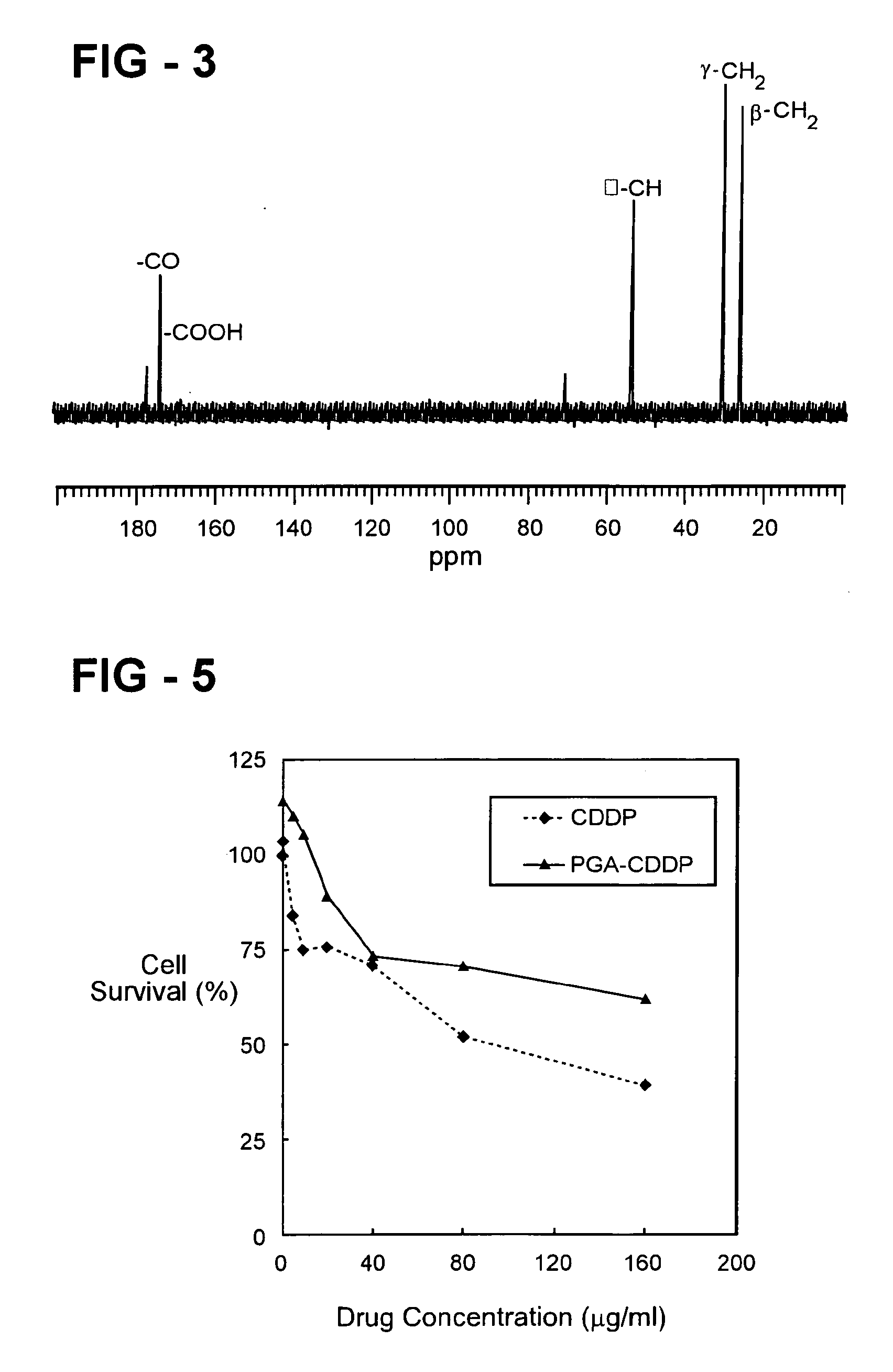
Preparation and applications of novel complexes made by gamma-polyglutamic acid and cisplatin
a technology of polyglutamic acid and complexes, applied in the field of complexes made from gamma-polyglutamic acid and cisplatin, can solve the problems of low yield, high production cost, and limit commercialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
(1) Method of Preparing γ-PGA-CDDP Complex
[0048]11.4 mg AgNO3 and 20 mg cisplatin were dissolved in 10 ml double distilled water. The resulting solution was allowed to sit for 24 h. then centrifuged to remove the precipitate and filtered through a 0.22 μm membrane to remove fine particulates. 75 mg of small molecule γ-PGA was added to the resulting solution and the pH was adjusted to 7-8. The resulting mixture was mixed at 37° C. for 48 h under no light and then dialyzed for 24 hr to remove un-reacted cisplatin. Lyophilize was used to obtain the γ-PGA-CDDP complex as a white crystalline powder. Based on this procedure, the mole ratio of cisplatin and γ-PGA is 25:1, or m=25. This method of preparing γ-PGA-CDDP complex was used in all of the following examples.
[0049]11.4 mg AgNO3 and 30 mg cisplatin were dissolved in 10 ml double distilled water and was allowed to sit for 24 h. then centrifuged to remove the precipitate and filtered through 0.22 μm membrane to remove fine particulates...
example 2
(1) Use Fermentation to Obtain Large Molecule γ-PGA
[0052]Activate Bacillus Licheniformis ATCC 9945a, was inoculated to a slant culture and incubated at 37° C. for 11 h. The culture includes peptone (10 g / l), NaCl (5 g / l), yeast extract (5 g / l), and agar (20 g / l).
[0053]Culturing: Inoculate the activated bacterial in the cell culture, 37° C., 210 rpm.
[0054]Culturing medium: peptone (10 g / l), NaCl (5 g / l), yeast extract (5 g / l).
[0055]Fermentation medium: peptone (50 g / l), yeast extract (10 g / l), glutamic acid (30 g / l), NaCl (10 g / l), KH2PO4 (5 g / l), and MgSO4.7H2O (0.5 g / l). The fermentation medium was used to fill in a 200 ml / 1000 ml shaking bottle and sterilized at 115° C. for 20 min. After sterilization and cool down, the medium was inoculated with 5% of Bacillus Licheniformis ATCC 9945a and cultured at 37° C. and rotated at 220 rpm. Fermentation was conducted for 48 h.
[0056]After fermentation, distilled water was added to the fermentation aliquot (4 times dilution), the pH was adju...
example 3
Large-Scale Preparation of Small Molecule γ-PGA
[0058]A 2% aqueous solution of the large molecule γ-PGA was prepared and the pH was adjusted to 2˜3. The aliquot was subjected to high temperature and high pressure (121° C., 0.1 MPa) for 15, 20, and 30 min and immediately placed in ice bath. The pH was adjusted to 7˜8. The aliquot was dialyzed and lyophilized to obtain small molecule γ-PGA as a white crystalline powder. An Ubbelohde viscometer to measure the molecular weight of the small molecule γ-PGA which was found to be 80-100 kD, 35-60 kD, or 5-20 kD. Preparation of the small molecule γ-PGA-CDDP complex was the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| physical | aaaaa | aaaaa |
| chemical properties | aaaaa | aaaaa |
| biological compatibility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com