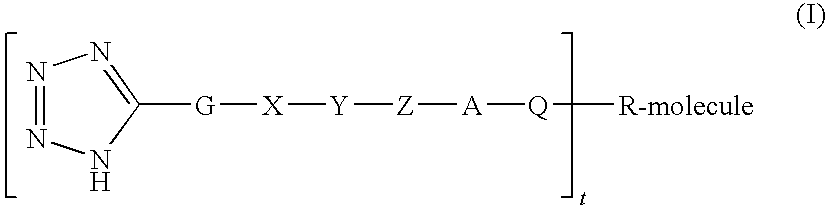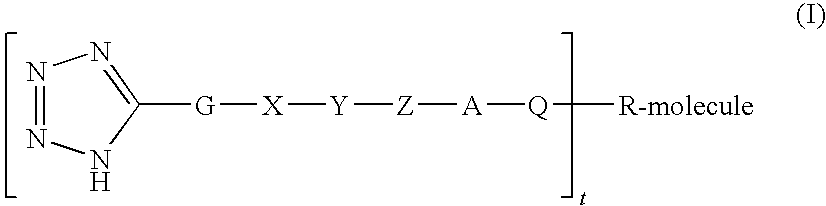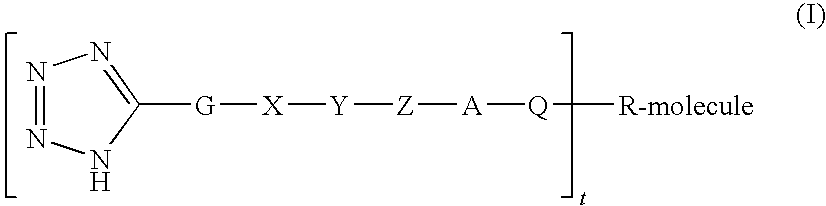Polypeptide protracting tags
a polypeptide and protracting tag technology, applied in the field of polypeptide protracting tags, can solve the problems of not being suitable for drug candidates, endogenous peptides are not always suitable for endogenous peptides, and the reason for the strong variability of the plasma half-live of peptides, proteins, or other compounds is not well understood, and achieve the effect of increasing the plasma half-life of a molecul
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
16-(5-tetrazolyl)hexadecanoic Acid
[0263]
[0264]A mixture of 16-bromohexadecanoic acid (16.61 g, 49.5 mmol), DMSO (150 ml), NaCN (12.5 g, 255 mmol), and NaI (1.92 g, 12.8 mmol) was stirred at 120° C. for 20 h. The mixture was allowed to cool to room temperature, and was then poured into a stirred mixture of water (1.7 l) and concentrated HCl (30 ml). Rinsing with water (100 ml). The resulting suspension was stirred at room temperature overnight. The product was filtered and washed with water (2×100 ml), and the solid was recrystallized twice from MeCN (90 ml and 50 ml). 10.1 g (72%) of 16-cyanohexadecanoic acid was obtained.
[0265]1H NMR (DMSO) δ 1.20-1.39 (m, 22H), 1.50 (m, 4H), 2.18 (t, J=7 Hz, 2H), 2.48 (t, J=7 Hz, 2H), 11.95 (s, 1H).
[0266]This product was mixed with DMF (150 ml), AcOH (10.0 ml, 174.8 mmol), NEt3 (25 ml, 180 mmol), and NaN3 (11.83 g, 182 mmol), and the mixture was stirred at 120° C. for 80 h, while following the conversion by 1H NMR. The mixture was concentrated und...
example 2
4-(N-(16-(5-tetrazolyl)hexadecanoyl)sulfamoyl)butyric Acid
[0268]
[0269]To a suspension of 16-(5-tetrazolyl)hexadecanoic acid (3.25 g, 10.0 mmol) in DCM (40 ml) was added oxalyl chloride (1.2 ml, 14.0 mmol). The mixture was stirred at room temperature for 42 h, concentrated, coevaporated once with PhMe, and to the residue were added a solution of methyl 4-sulfamoyl butyrate (1.66 g, 9.16 mmol) in DCM (35 ml) and then DMAP (3.67 g, 30.0 mmol). The heterogenous mixture was stirred at room temperature for 6.5 h and then concentrated. To the residue was added a mixture of water (50 ml) and 1N HCl (50 ml), and the resulting mixture was stirred at room temperature for 5 d. The product was filtered, washed with water (100 ml), and recyrstallized from MeCN (25 ml), to yield 1.84 g (41%) of the N-acylsulfonamide methyl ester. To this ester (1.06 g, 2.17 mmol) in MeOH (15 ml) was added a solution of NaOH (0.38 g, 9.5 mmol, 4.4 eq) in water (1.5 ml). After stirring at room temperature for 1.5 h ...
example 3
16-(4′-(5-tetrazolyl)biphenyl-4-yloxy)hexadecanoic Acid
[0271]
16-Bromohexadecanoic Acid Methyl Ester
[0272]A mixture of 16-bromohexadecanoic acid (15.5 g, 46.2 mmol), MeOH (100 ml), PhMe (30 ml), trimethylorthoformate (30 ml), and polystyrene-bound benzenesulfonic acid (3.6 g) was stirred at 55° C. After 69 h the mixture was filtered through celite and the filtrate was concentrated to yield 16.85 g of an oil (100% yield).
(4′-Cyanobiphenyl-4-yloxy)hexadecanoic Acid Methyl Ester
[0273]A mixture of 16-bromohexadecanoic acid methyl ester (4.86 g, 13.9 mmol), MeCN (20 ml), 4-cyano-4′-hydroxybiphenyl (3.16 g, 16.2 mmol), and K2CO3 (2.45 g, 17.7 mmol) was stirred at 82° C. After 17 h satd aquous NaHCO3 (150 ml) was added, and the product was filtered, washed with water, and recrystallized from boiling MeCN (approx 80 ml). Filtration and drying under reduced pressure yielded 5.40 g (84%) of (4′-cyanobiphenyl-4-yloxy)hexadecanoic acid methyl ester colorless needles.
16-(4′-(5-Tetrazolyl)biphenyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com