Cell nuclear transfer
a cell nuclear and transfer method technology, applied in the field of cell nuclear transfer in mammals, can solve the problems of high cost, high cost, time-consuming work, etc., and achieve the effect of low buoyant densities of porcine oocytes, high cost, and high cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
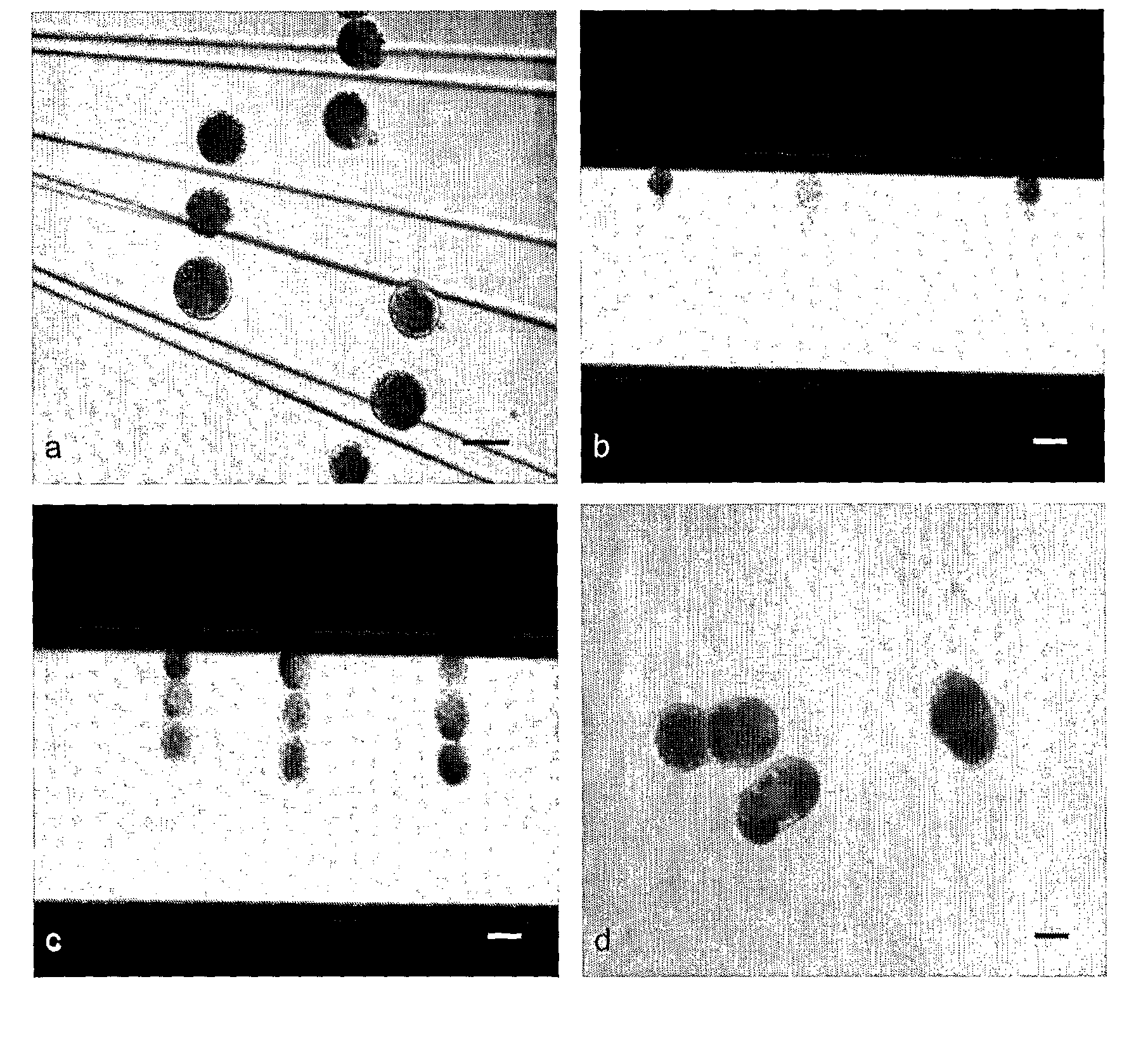
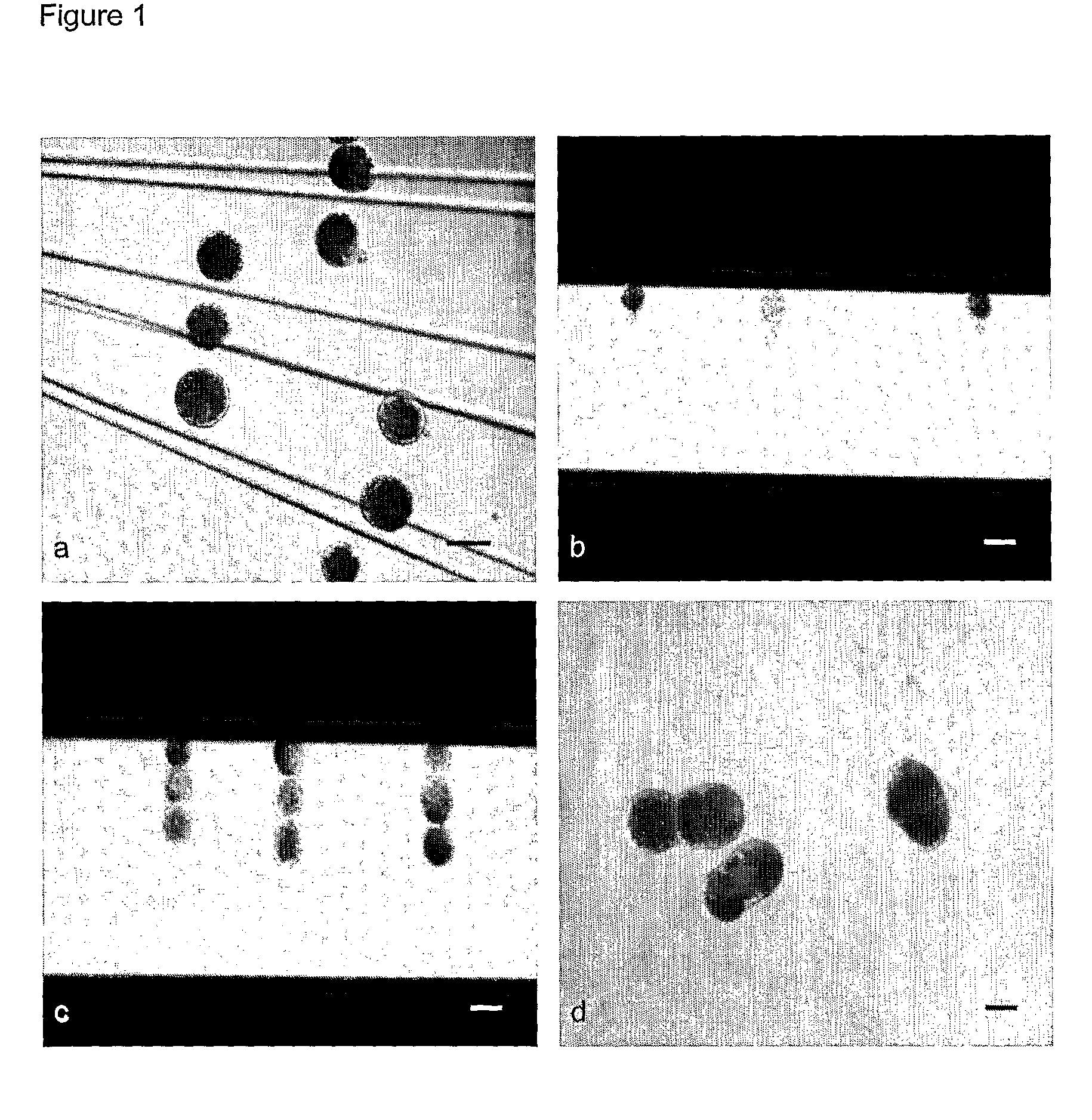
[0160]Differences in developmental competence between sow (2.5 years, 170 Kg in weight) derived oocytes and gilt (5.5˜6 months, 75 Kg in weight) derived oocytes were investigated through ZF and ZI PA after 44 h in vitro maturation. Four combined groups were investigated in 3 identical replicates: (1) ZF oocytes from sows (2) ZI oocytes from sows (3) ZF oocytes from gilts (4) ZI oocytes from gilts. For ZF activation, a single DC pulse of 0.85 KV / cm for 80 μs was applied, while a single 1.25 KV / cm pulse was used to activate ZI oocytes. Following 7 days culture as described above, the percentage of blastocysts per activated embryo was determined.
[0161]The in vitro developmental competence of parthenogenetically activated oocytes derived from either sows or gilts was investigated. As shown in Table 1, the blastocyst rates of parthenogenetically activated oocytes from sows were significantly higher than those from gilts, either after ZF or ZI PA.
TABLE 1Blastocyst development of Day 7 par...
example 2
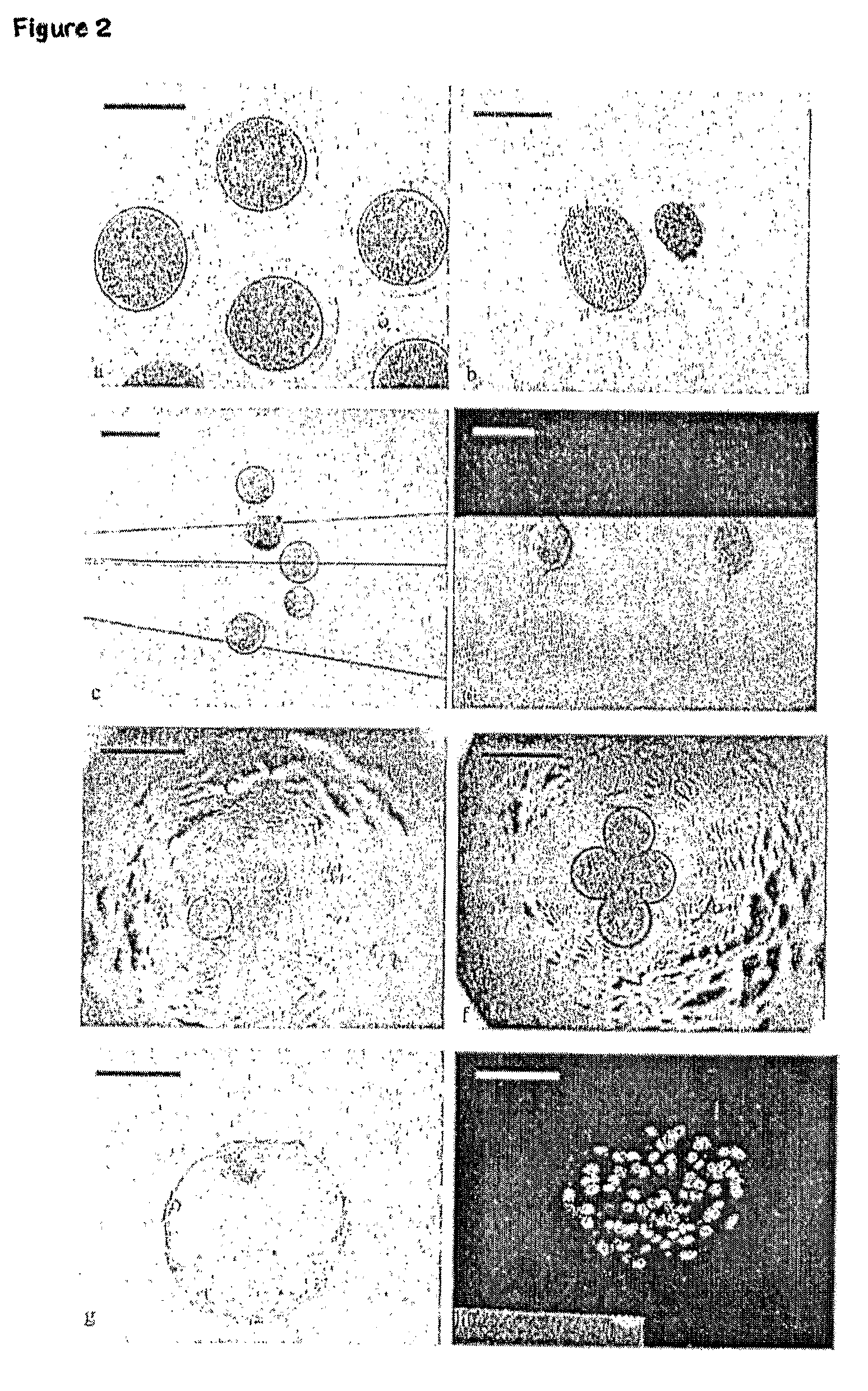
[0163]The feasibility of modified porcine HMC was investigated in 6 identical replicates, with IVF and in parallel ZF PA as controls. The more competent sow oocytes (according to Example 1) were used in Example 2. Seven days after reconstruction and / or activation, the number of blastocysts per reconstructed embryo and total cell numbers of randomly selected blastocysts were determined.
[0164]More than 90% of oocyte fragments derived from morphologically intact oocytes could be recovered for HMC after the trisection. In average, 37 embryos could be reconstructed out of 100 matured oocytes. The developmental competence of all sources of porcine embryos is shown in Table 2. On Day 7, the development of reconstructed embryos to the blastocyst stage was 17±4% with mean cell number of 46±5, while the blastocyst rates for IVF, and ZF PA were 30±6% and 47±4% (n=243, 170, 97) respectively.
TABLE 2In vitro development of embryosproduced by HMC, IVF and ZF PANo. ofblastocystMean cellEmbryoembryo...
example 3
[0167]Vitrification of hand-made cloned porcine blastocysts produced from delipated in vitro matured oocytes.
[0168]Recently a noninvasive procedure was published for delipation of porcine embryos with centrifugation but without subsequent micromanipulation (Esaki et al. 2004 Biol Reprod. 71, 432-6).
[0169]Cryopreservation of embryos / blastocysts was carried out by vitrification using Cryotop (Kitazato Supply Co, Fujinomiya Japan) as described previously (Kuwayama et al. 2005a; 2005b). At the time of vitrification, embryos / blastocysts were transferred into equilibration solution (ES) consisting of 7.5% (V / V) ethylene glycol (EG) and 7.5% dimethylsulfoxide (DMSO) in TCM199 supplemented with 20% synthetic serum substitute (SSS) at 39° C. for 5 to 15 min. After an initial shrinkage, embryos regained their original volume. 4˜6 embryos / blastocysts were transferred into 20 ul drop of vitrification solution (VS) consisting of 15% (V / V) EG and 15% (DMSO) and 0.5M sucrose dissolved in TCM199 su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com