Therapeutic Gene-Switch Constructs and Bioreactors for the Expression of Biotherapeutic Molecules, and Uses Thereof
a technology of biotherapeutic molecules and constructs, which is applied in the direction of vectors, vector-based foreign material introduction, dna/rna fragmentation, etc., can solve the problems of genetic material entering, protein secretion cannot be regulated, and hinder the progress of successful therapies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
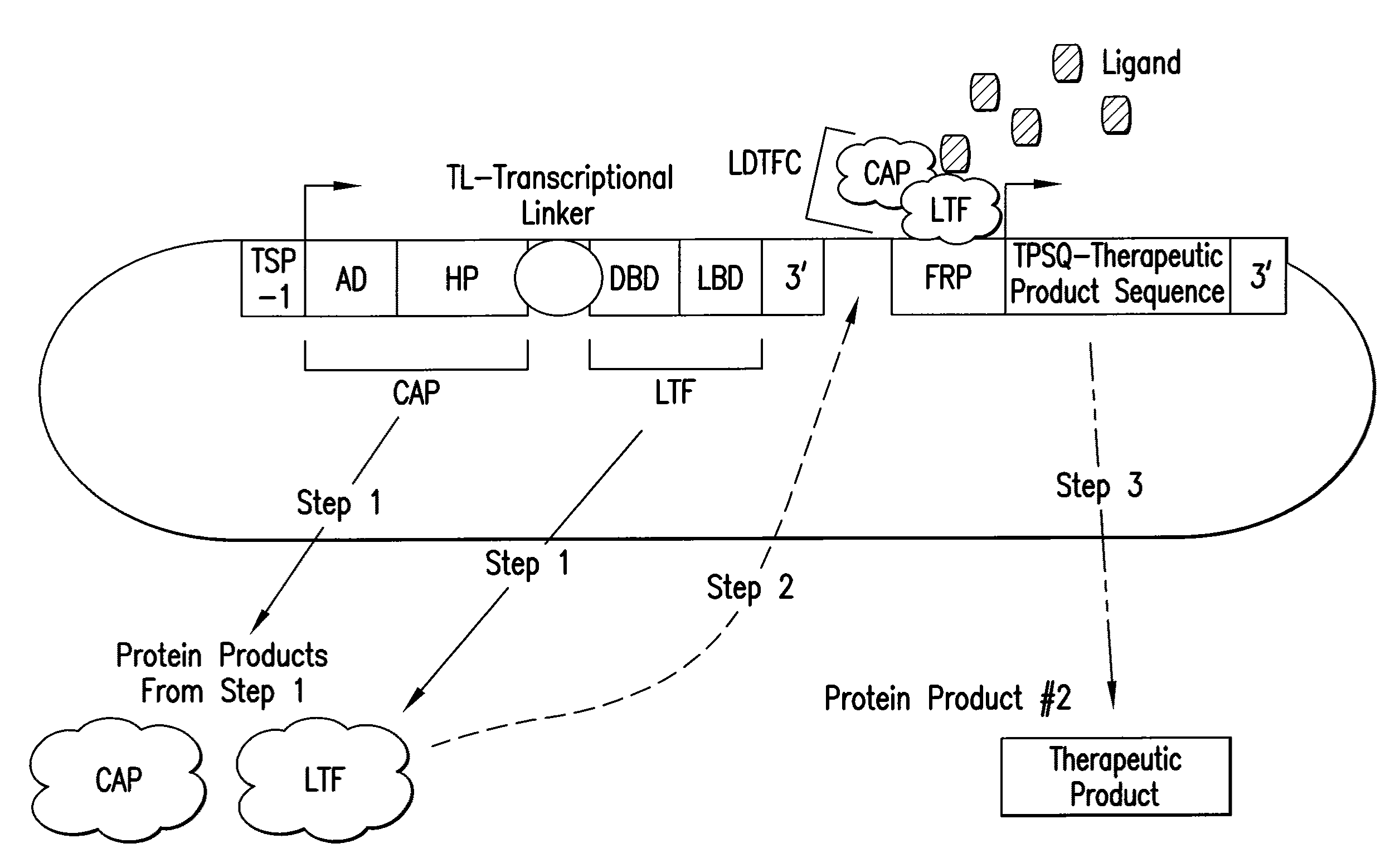
[0409]This example describes a gene therapy vector useful for the treatment of ischemic heart disease through the promotion of angiogenesis. Insulin like growth factor 1 is a hormone that may be useful in the treatment of ischemic heart disease. (IGF-1, GenBank Accession No.: NP—001104753.1, SEQ ID NO:20). Use of IGF-1 in preclinical models is associated with improved cardiac function, anti-apoptosis, neo-vascularization and cardiac muscle regeneration (reviewed in Santini, M. P., et al. Novartis Found Symp. 274:228-38 (2006); discussion 239-43, 272-6; and Saetrum Opgaard, O., and Wang, P. H. Growth Horm IGF Res. 15:89-94 (2005)). For this purpose, an example of inducible IGF-1 expression, in response to ischemia and / or inflammation is given. An inducible expression system for the expression if IGF-1 upon administration of ligand, under hypoxic conditions which occur in ischemic tissue is shown in FIG. 5.
SEQ ID NO: 20:MGKISSLPTQLFKCCFCDFLKVKMHTMSSSHLFYLALCLLTFTSSATAGPETLCGAELVDALQFV...
example 2
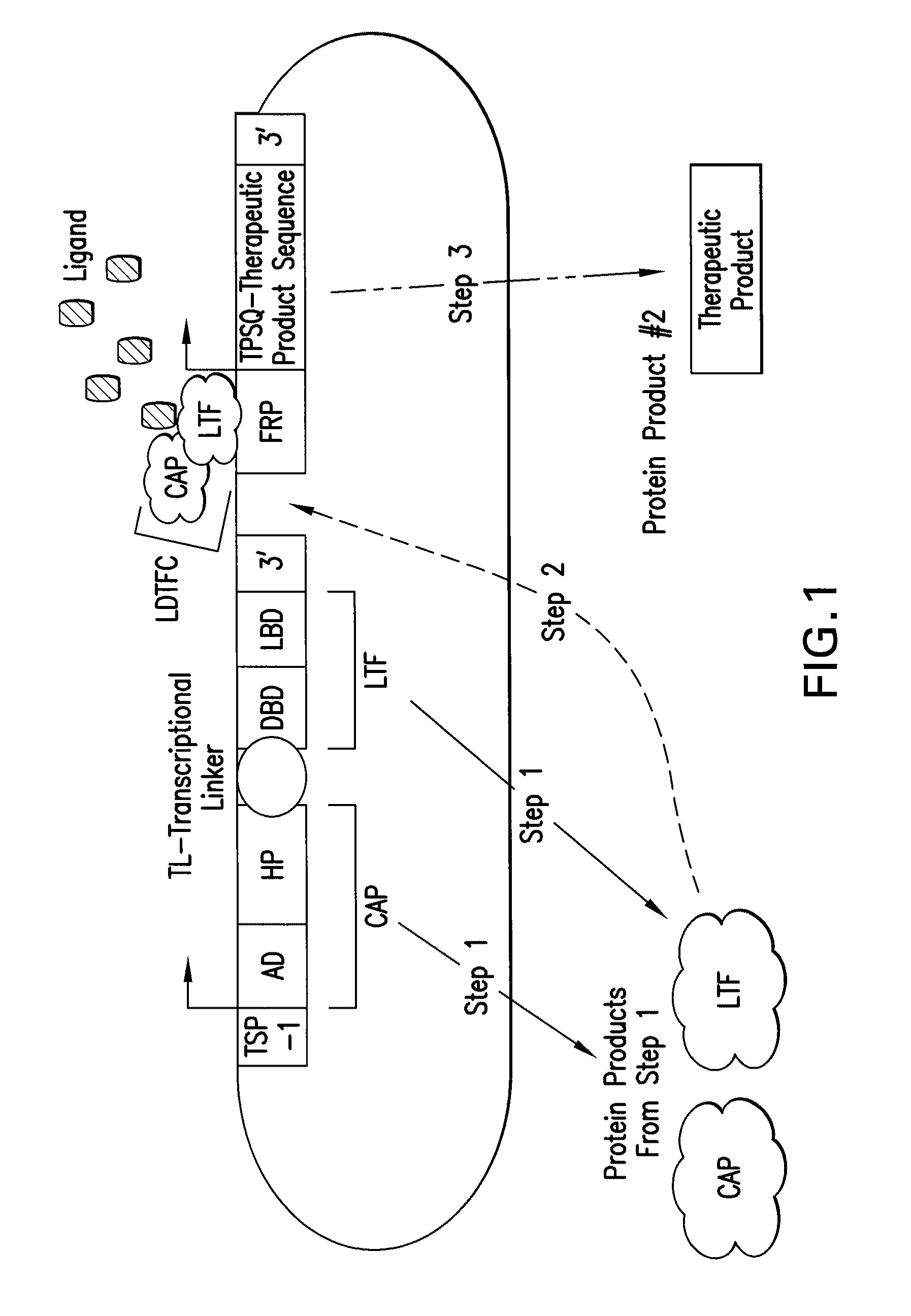
[0415]This example describes a bioreactor / cell therapy vector useful for the treatment of ischemic cardiovascular disease through the promotion of angiogenesis and cardioprotection. The vector, shown in FIG. 6, will confer expression of human basic fibroblast growth factor (bFGF, GenBank Accession No.: NP—001997, SEQ ID NO:21) upon administration of ligand, under hypoxic conditions which occur in ischemic tissue.
SEQ ID NO: 21:MVGVGGGDVEDVTPRPGGCQISGRGARGCNGIPGAAAWEAALPRRRPRRHPSVNPRSRAAGSPRTRGRRTEERPSGSRLGDRGRGRALPGGRLGGRGRGRAPERVGGRGRGRGTAAPRAAPAARGSRPGPAGTMAAGSITTLPALPEDGGSGAFPPGHFKDPKRLYCKNGGFFLRIHPDGRVDGVREKSDPHIKLQLQAEERGVVSIKGVCANRYLAMKEDGRLLASKCVTDECFFFERLESNNYNTYRSRKYTSWYVALKRTGQYKLGSKTGPGQKAILFLPMSAKS
[0416]The complete nucleotide sequence of the construct shown in FIG. 6 is presented as SEQ ID NO:8. The nucleotide coordinates for salient elements of the construct are shown in Table 5.
TABLE 5LabelDirectionLengthStartEnd3′Reg(HSVTKpA)reverse259318576Neoreverse7955831377SV40 ea...
example 3
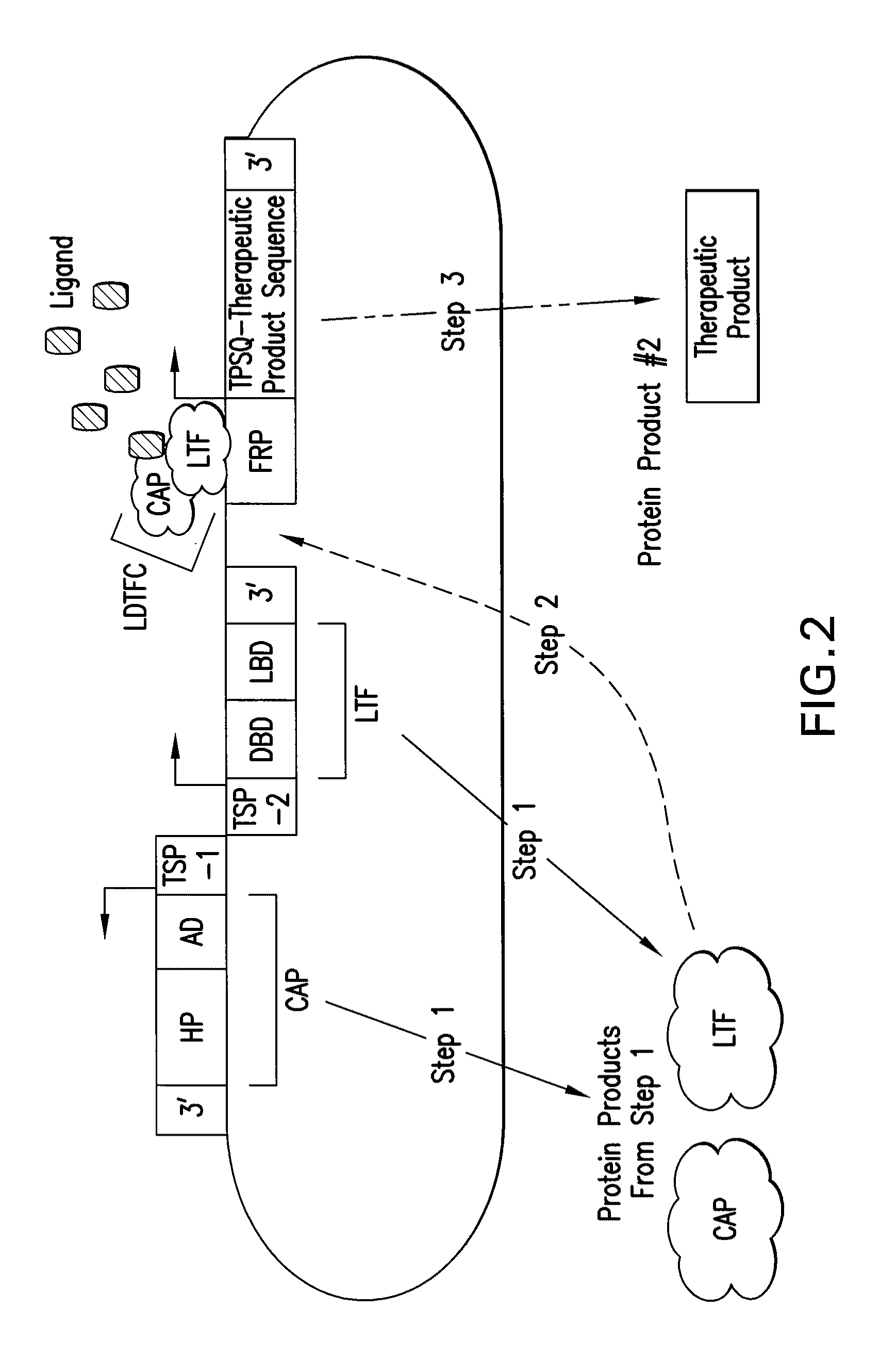
[0421]This example describes a bioreactor / cell therapy vector useful for the treatment of ischemic cardiovascular disease through the promotion of cardioprotection. The vector, shown in FIG. 7, will confer expression of human erythropoietin (EPO, GenBank Accession No.: CAA26095.1, SEQ ID NO:22) upon administration of ligand, under hypoxic conditions which occur in ischemic tissue. Erythropoietin has been shown to function in cardioprotection and anti-remodeling, in response to ischemia.
SEQ ID NO: 22:MGVHECPAWLWLLLSLLSLPLGLPVLGAPPRLICDSRVLQRYLLEAKEAENITTGCAEHCSLNENITVPDTKVNFYAWKRMEVGQQAVEVWQGLALLSEAVLRGQALLVNSSQPWEPLQLHVDKAVSGLRSLTTLLRALGAQKEAISPPDAASAAPLRTITADTFRKLFRVYSNFLRGKLKLYTGEACRTGDR
[0422]The complete nucleotide sequence of the construct shown in FIG. 7 is presented as SEQ ID NO:9. The nucleotide coordinates for salient elements of the construct are shown in Table 6.
TABLE 6LabelDirectionLengthStartEnd3′Reg(HSVTKpA)reverse259318576Neoreverse7955831377SV40 early promoterreverse2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore sizes | aaaaa | aaaaa |
| semi-permeable | aaaaa | aaaaa |
| nucleic acid | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com