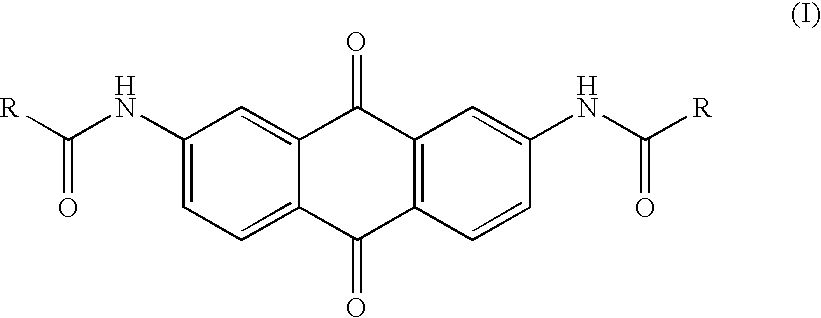
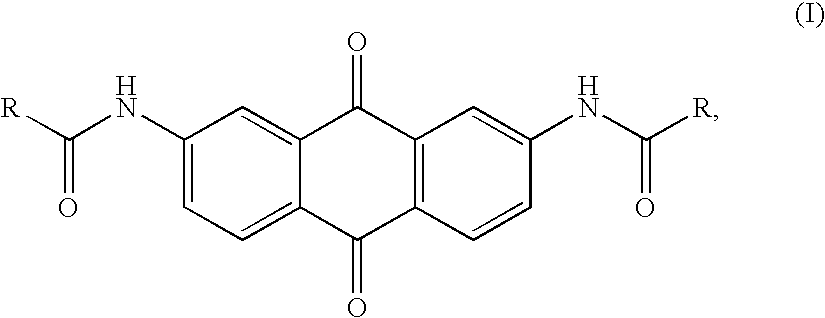
Synthesis, telomerase inhibition and cytotoxic studies on 2,7-disubstituted anthraquinone derivatives
a technology of telomerase and anthraquinone, which is applied in the field of anthraquinone compound having cytotoxicity, can solve the problems of inability to achieve the abovementioned aspects, cell end-replication problem,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030]The present invention will now be described more specifically with reference to the following Embodiments. It is to be noted that the following descriptions of preferred Embodiments of this invention are presented herein for purpose of illustration and description only; it is not intended to be exhaustive or to be limited to the precise form disclosed.
[0031]The anthraquinone compounds disclosed in the present invention mainly are 2,7-diaminoanthraquinone and its derivatives, and the preferred embodiments of the synthetic method thereof are described as follows.
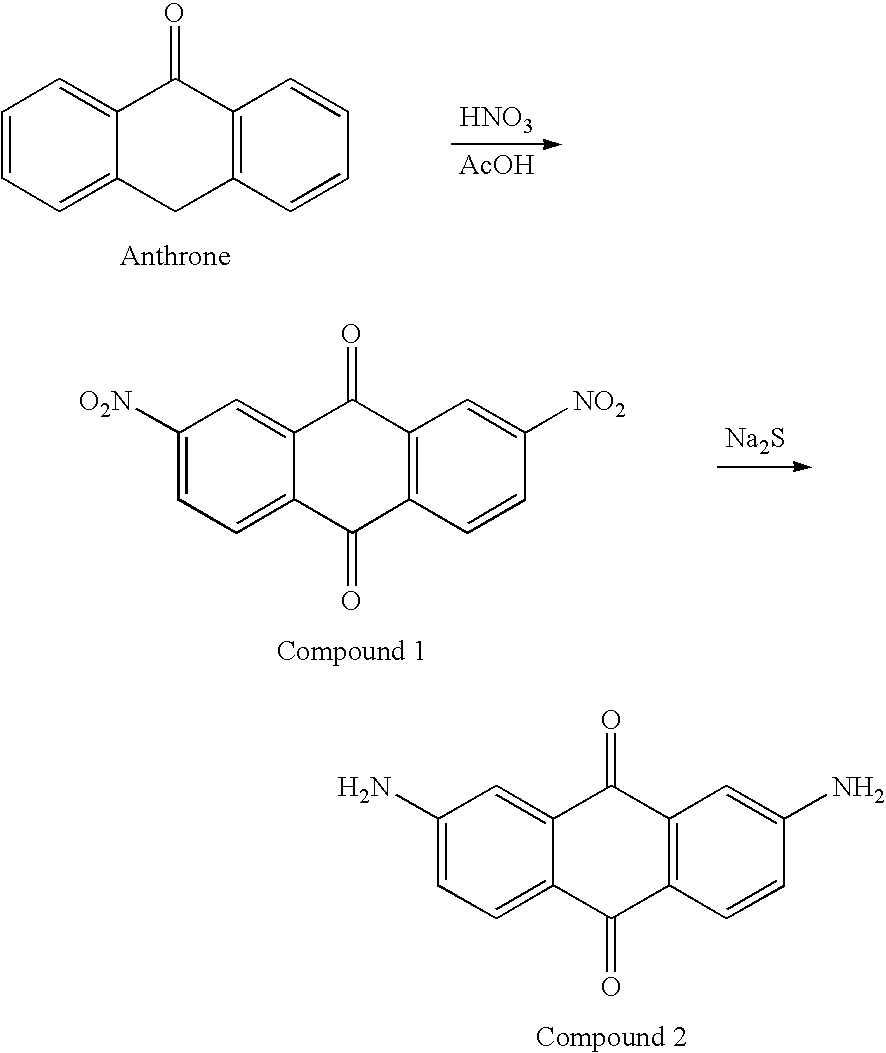
I. Synthesis of 2,7-diaminoanthraquinone
[0032]
[0033]In the present invention, anthrone is firstly oxidized and nitrified, and the nitro group (—NO2) of the aforementioned compound is reduced as amino group (—NH2) with sodium sulfide. The obtained compound then is being the starting material of the series synthetic derivatives of the present invention. The detailed steps are described as follows.
[0034]Anthrone of 0.01 mol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com