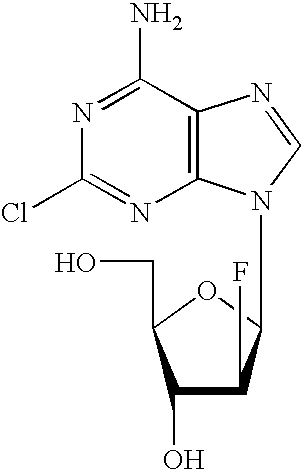
Methods and Compositions for the Treatment of Lupus Using Clofarabine
a technology of lupus and compositions, applied in the field of methods and compositions for the treatment of lupus using clofarabine, can solve the problems of suppressing the immune system's ability to fight infection, worsening the course of the disease, and reducing the effect of the spread or worsening of the diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
5.1 Example 1
Parenteral Dosage Formulation
[0105]Clofarabine, 9.86 g, is wetted / partially dissolved with 600 mL of a 9:1 mixture of tertiary butanol and Water for Injection USP which is pre-cooled to 5° C. Once the drug powder is completely wetted, dissolution is completed by the addition of 600 mL of a 1:9 mixture of tertiary butanol and Water for Injection and 766 mL of a 1:1 mixture of tertiary butanol and Water for Injection which likewise is pre-cooled to 5° C. thereby making the final solution a 1:1 mixture. The dissolution is carried out under protection from light.
[0106]The solution formed above is promptly lyophilized in a Virtis INOTOP lyophilizer at −16° C. under light protectant conditions over a period of 48 hours. The resultant lyophilized product (lyophile) is then further dried at 15° C. under high vacuum for 48 hours. No detectable degradation of the drug is observed during these procedures. The lyophile is packaged under sterile conditions into 30 mL vials, each con...
example 2
5.2 Example 2
25 mg Dosage Capsule
[0108]Table 1 illustrates a batch formulation and a single dose unit formulation containing 25 mg of Clofarabine.
TABLE 1Formulation for 25 mg tabletPercentQuantityQuantityMaterialby Weight(mg / tablet)(kg / batch)Clofarabine 40%25.0020.00Microcrystalline53.5% 33.4426.75Cellulose, NFPluronic F-684.0%2.502.00SurfactantCroscarmellose2.0%1.251.00Sodium Type A, NFMagnesium Stearate, NF0.5%0.31250.25Total100.0% 62.50 mg50.00 kg
[0109]The microcrystalline cellulose, croscarmellose sodium, and Clofarabine components are passed through a #30 mesh screen (about 430μ to about 655μ). The Pluronic F-68® (manufactured by JRH Biosciences, Inc. of Lenexa, Kans.) surfactant is passed through a #20 mesh screen (about 457μ to about 1041μ). The Pluronic F-68® surfactant and 0.5 kgs of croscarmellose sodium are loaded into a 16 qt. twin shell tumble blender and are mixed for about 5 minutes. The mix is then transferred to a 3 cubic foot twin shell tumble blender where the mic...
example 4
5.4 Example 4
200 mg Dosage Capsule
[0113]Table 3 illustrates a batch formulation and single dosage formulation for a 200 mg Clofarabine single dose unit, i.e., about 40 percent by weight.
TABLE 3Formulation for 200 mg capsulePercentQuantityQuantityMaterialBy Weight(mg / tablet)(kg / batch)Clofarabine40.0%200mg16.80 kgPregelatinized Corn9.5%297.5mg24.99 kgStarch, NF5Magnesium Stearate0.5%2.5mg 0.21 kgTotal100.0%500mg42.00 kg
[0114]The pregelatinized corn starch (SPRESS B-820) and 3-[2-(3′-methyl-biphen-4-yloxy)-acetylamino]-benzoic acid components are passed through a 710 μm screen and then are loaded into a Diffusion Mixer with a baffle insert and blended for 15 minutes. The magnesium stearate is passed through a 210 μm screen and is added to the Diffusion Mixer. The blend is then encapsulated in a size #0 capsule, 500 mg per capsule (8400 capsule batch size) using a Dosator type capsule filling machine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com