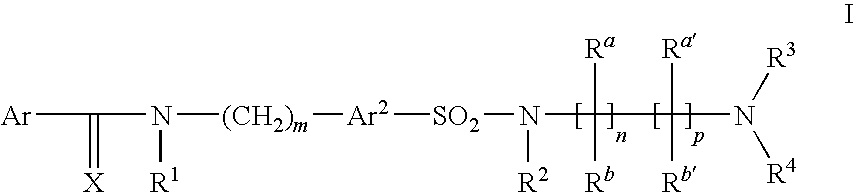
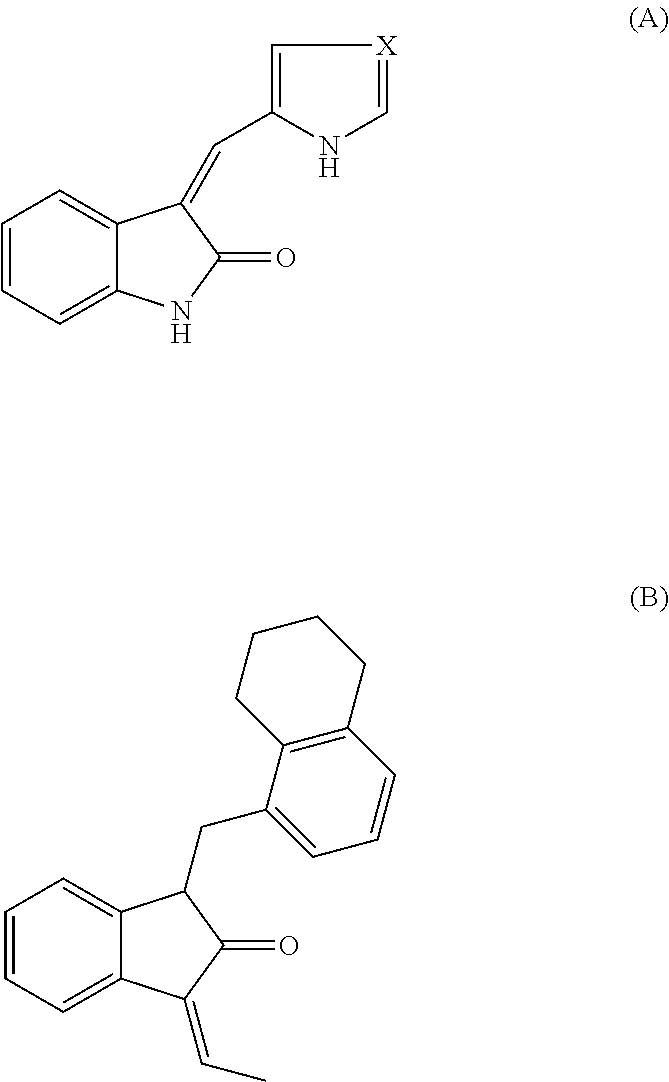
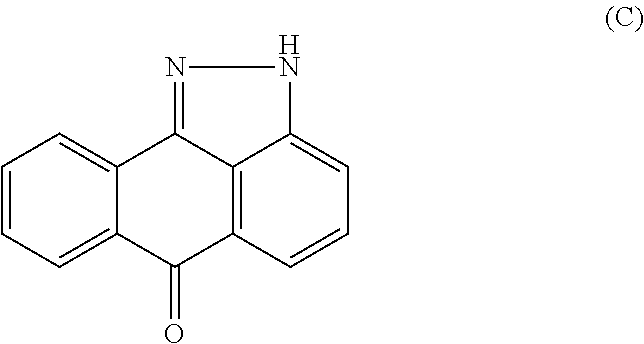
Arylsulfonamide derivatives as c-jun-n-terminal kinases (jnk's) inhibitors
a technology of jun-n-terminal kinase and derivatives, which is applied in the field of arylsulfonamide derivatives, can solve the problems of defective regulation of the growth of the blood vessel wall, cell death and scar formation, renal failure or cerebral dysfunction,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0123]The invention will be illustrated by means of the following examples which are not to be construed as limiting the scope of the invention.
[0124]The compounds of the present invention may be synthesized according to the different synthesis pathways provided above. The following examples illustrate preferred methods for synthesizing the compounds according to formula I and determining their activities.
example i
5-({[1-(4-Chloro-phenyl)-methanoyl]-amino}-methyl)-thiophene-2-sulfonyl chloride (1b) (compound of formula III)
a) 4-Chloro-N-thiophen-2-ylmethyl-benzamide (1a)
[0125]
[0126]A solution of 4-chlorobenzoyl chloride (0.114 mol) in 50 mL dry CH2Cl2 was added over 30 min to a stirred solution of 2-aminomethyl-thiophene (0.137 mol) and iPr2NEt (0.25 mol) in CH2Cl2 (200 mL) at 0° C. A white solid was formed and the reaction was allowed to warm to room temperature over 1 h. The mixture was diluted with 200 mL of CH2Cl2, washed twice with HCl aq. (0.1N) and dried over MgSO4. Evaporation of the solvents afforded 28 g (98%) of the title benzamide (1a) as a white solid: m.p. 153-54° C., 1H NMR (CDCl3) δ 7.9 (d, J=8.67 Hz, 2H), 7.58 (d, J=8.67 Hz, 2H), 7.44 (dd, J=3.77, 1.13 Hz, 1H), 7.22 (d, J=5.27 Hz, 1H), 7.16 (dd, J=3.39, 5.27 Hz, 1H), 6.62 (br d, 1H), 4.98 (d, J=5.65 Hz, 2H).
b) 5-({[1-(4-Chloro-phenyl)-methanoyl]-amino}-methyl)-thiophene-2-sulfonyl chloride (1b)
[0127]
[0128]Chlorosulfonic acid ...
example ii
4-Chloro-N-{5-[1-(4-trifluoromethyl-benzyl)-piperidin-3-ylsulfamoyl]-thiophen-2-ylmethyl}-benzamide (2)
[0129]The synthesis of the above compound (2) is a 3-step-synthesis (see scheme I).
Protocol I:
Step 1-N-Sulfonylation (Compound of Formula IV)
[0130]The mono protected diamine (+ / −)-3-Amino-1-N-Boc-Piperidine (compound of formula II) (0.4 g, 2 mMol, 1 eq), 5-(4-chlorobenzamidomethyl)thiophene-2-sulphonyl chloride (1b) (compound of formula III) (0.99 g, 2.4 mMol, 1.2 eq), and piperidine resin (2 g, 1.5 eq, loading of 1.5 mMol / g) are swirled in THF (50 ml) on orbital shaker overnight. Aminomethyl polystyrene (1.82 g, 1 eq, loading of 1.1 mMol / g) is added to the flask and contents swirled on orbital shaker overnight.
[0131]The resins are filtered and washed with a further 50 ml of THF. Filtrates are combined and solvent is evaporated under reduced pressure to yield quantitatively the corresponding sulfonamide (formula IV). No further purification is required at this stage.
Step 2—Removal ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com