Nanoemulsion therapeutic compositions and methods of using the same
a technology of nanoemulsions and compositions, applied in the direction of drug compositions, antibacterial agents, peptide/protein ingredients, etc., can solve the problems of pseudomonas aeruginosa, opportunistic and/or pathogenic bacteria infection, and is a major problem in the developed and undeveloped parts of the world
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
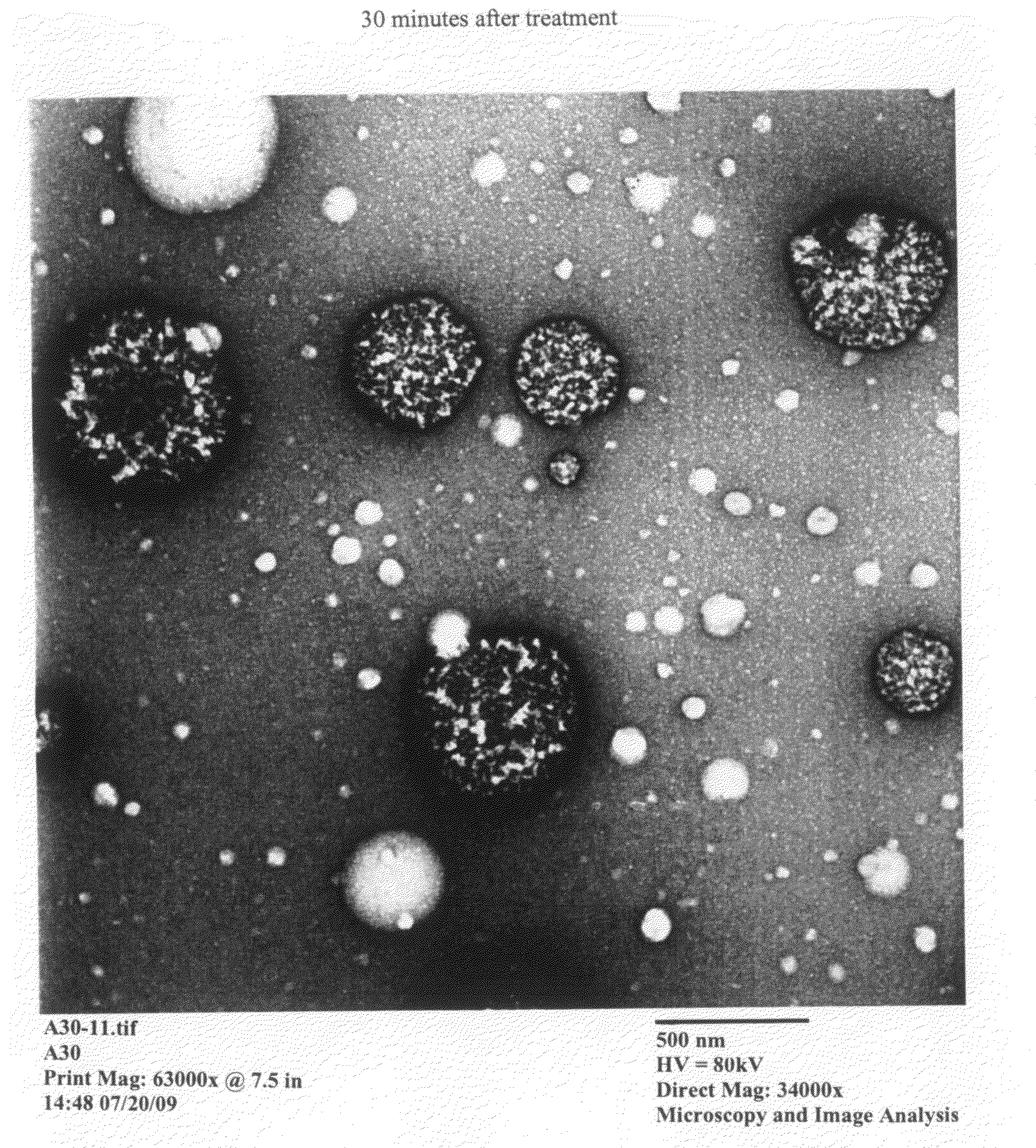
[0364]A nanoemulsion composition of the present invention was tested to determine if pulmonary administration of the composition to a subject would elicit any histological changes and / or pathology.
[0365]A solution comprising 20% nanoemulsion (W805EC) and 0.1% EDTA was administered via a nebulizer. A custom murine nose only nebulization chamber with PARI LC nebulizer (Midlothian, Va.) and compressor was used. A 0.9% NaCl solution was used as a control. Using the nebulizer, mice were administered nebulized nanoemulsion+EDTA or NaCl solution for 10 minutes. Twenty-four hours after administration, mice were sacrificed and physical properties and histology assessed.
[0366]Upon examination, there was an absence of histological changes indicating the absence of toxicity upon administration of nebulized nanoemulsion. Physical findings were normal.
example 2
Killing Assays Utilizing Nanoemulsion Compositions and Bacteria Found in the Respiratory Tract
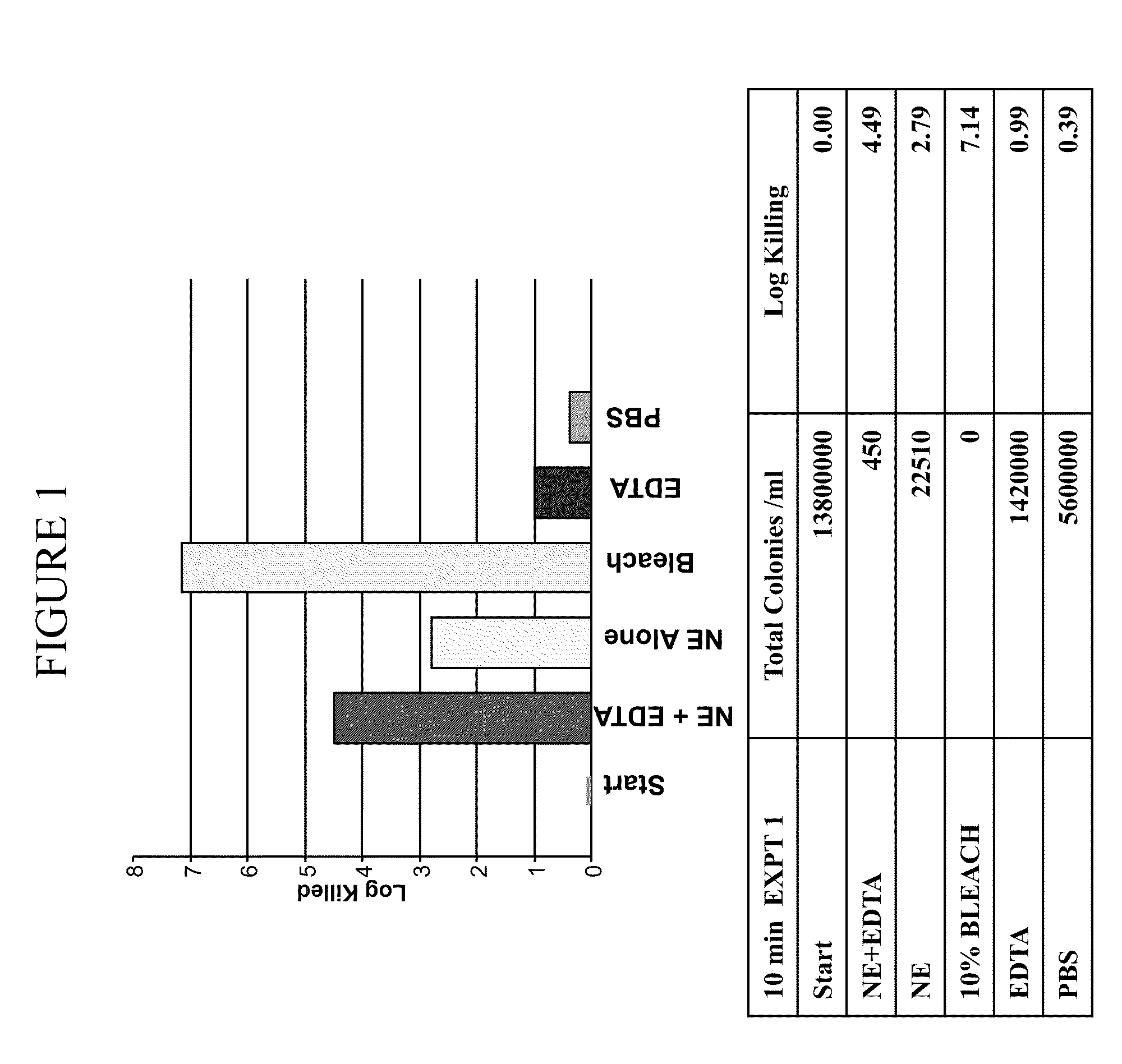
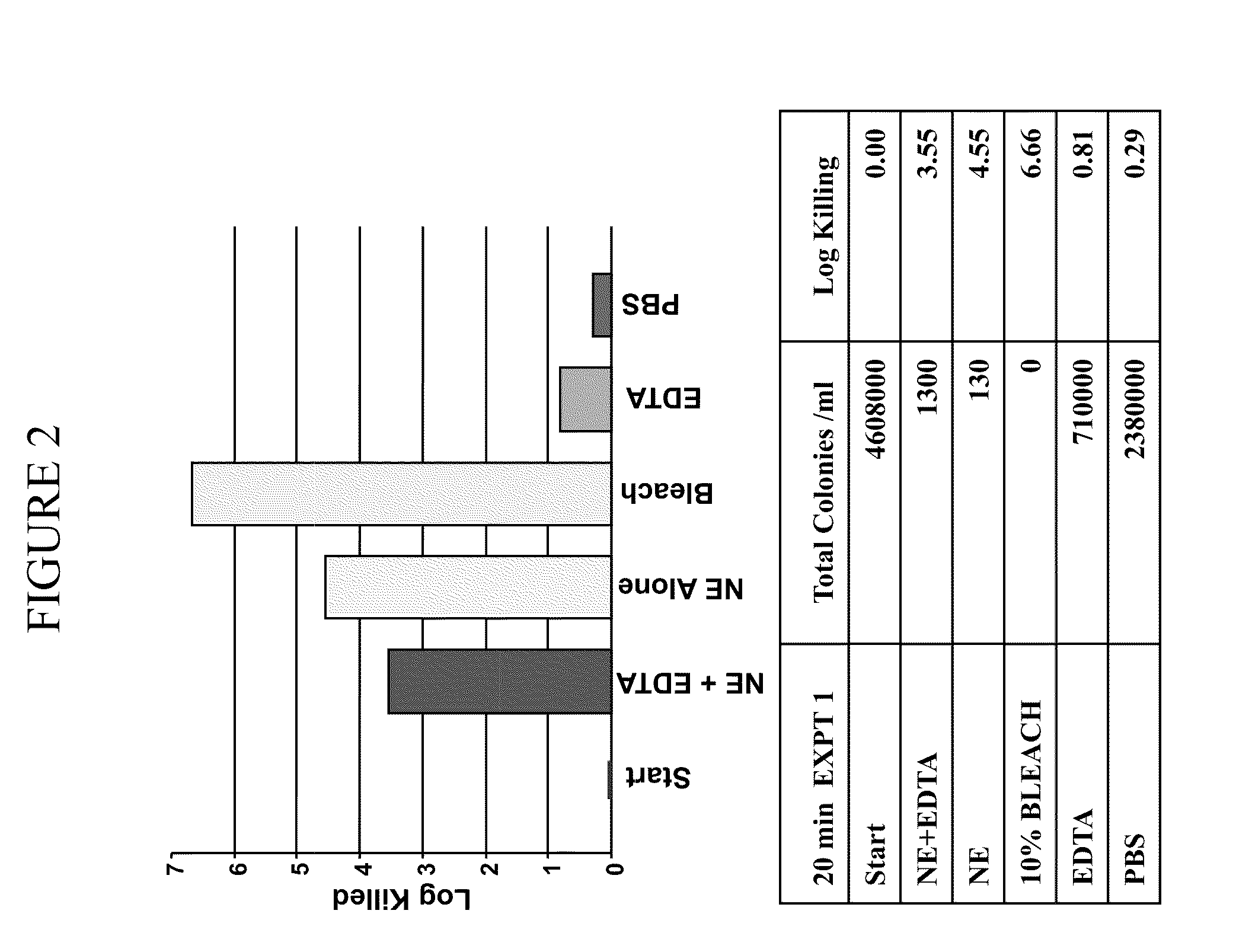
[0367]It was determined whether a composition comprising nanoemulsion (W805EC) alone or in combination with EDTA and / or the presence of a hypertonic salt solution would be able to attenuate growth and / or kill bacteria found in the respiratory tract.
[0368]An overnight culture of Burkholderia cepacia or Pseudomonas aeruginosa was started in 6 ml of cation adjusted Mueller Hinton Broth (MHB) from a frozen bacterial stock at −80° C. The culture was incubated in a shaking incubator at 37° C. The following day, bacteria were brought to logarithmic growth phase by back diluting the overnight culture 1:4 with fresh MHB. Back diluted culture was incubated at 37° C. in the shaking incubator until the OD600 reached between 0.40 to 0.45. One ml of the culture was spun down at 3500 to 4000 rpm for 15 minutes. The bacterial pellet was resuspended in 1 ml of sterile 2×PBS, 12% or 14% saline. Appropriate d...
example 3
Nanoemulsion Killing of Cystic Fibrosis (CF) Related Bacteria Including Multi-Drug Resistant Strains
[0378]Materials and Methods.
[0379]Bacterial strains and culture conditions. One hundred fifty isolates were analyzed including 75 Burkholderia isolates and 75 isolates belonging to other CF-relevant species, including Pseudomonas aeruginosa, Achromobacter xylosoxidans, Stenotrophomonas maltophilia, Acinetobacter species, Pandoraea species (P. apista, P. pnomenusa, P. pulmonicola, P. norimburgensis, and P. sputorum), and Ralstonia species (R. mannitolilytica and R. pickettii). One hundred forty-five clinical isolates were obtained from the Burkholderia cepacia Research Laboratory and Repository (BcRLR, University of Michigan, Ann Arbor, Mich.). These were recovered from 142 individuals between September 1997 and October 2007 and were referred to the BcRLR from 62 CF treatment centers in the U.S. for analysis. The remaining five strains included environmental isolates B. multivorans ATC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com