Positive electrode material for a lithium ion accumulator
a lithium ion accumulator and positive electrode technology, applied in the direction of conductors, cell components, nickel compounds, etc., can solve the problems of significant and sudden temperature rise, degradation of active materials, and the type of active materials that do not have sufficient heat stability, etc., to achieve good electrochemical power performance, good heat stability, and high electrochemical capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0064]Several compounds are made according to the synthesis method described earlier. The synthesized compounds are indicated in the Table 1 below.
TABLE 1Tested compoundsLi / MExample No.abcyxzNia(1−x)Mnb(1−x)Coc(1−x)Aly(1−x)Li(1+x)(1 + x) / (1 − x)10.400.400.200.000.0380.10.3850.38480.192—1.0381.08Comparative20.400.400.180.020.0380.10.3850.3850.1730.0191.0381.0830.400.400.150.050.0380.10.3850.3850.1440.0481.0381.0840.400.400.100.100.0380.10.3850.3850.0960.0961.0381.0850.4250.4250.100.050.0990.0750.4210.4210.0990.0961.0991.02Comparative60.400.400.100.100.0610.10.3760.3760.0940.0961.0611.1370.400.400.150.050.1070.10.3570.3570.1340.0481.1071.2480.4250.4250.150.000.0240.0750.4150.4150.146—1.0241.05Comparative90.4250.4250.100.050.0240.0750.4150.4150.0980.0481.0241.05100.4250.4250.050.100.0240.0750.4150.4150.0490.0961.0241.05110.800.000.150.05——0.048——Comparative
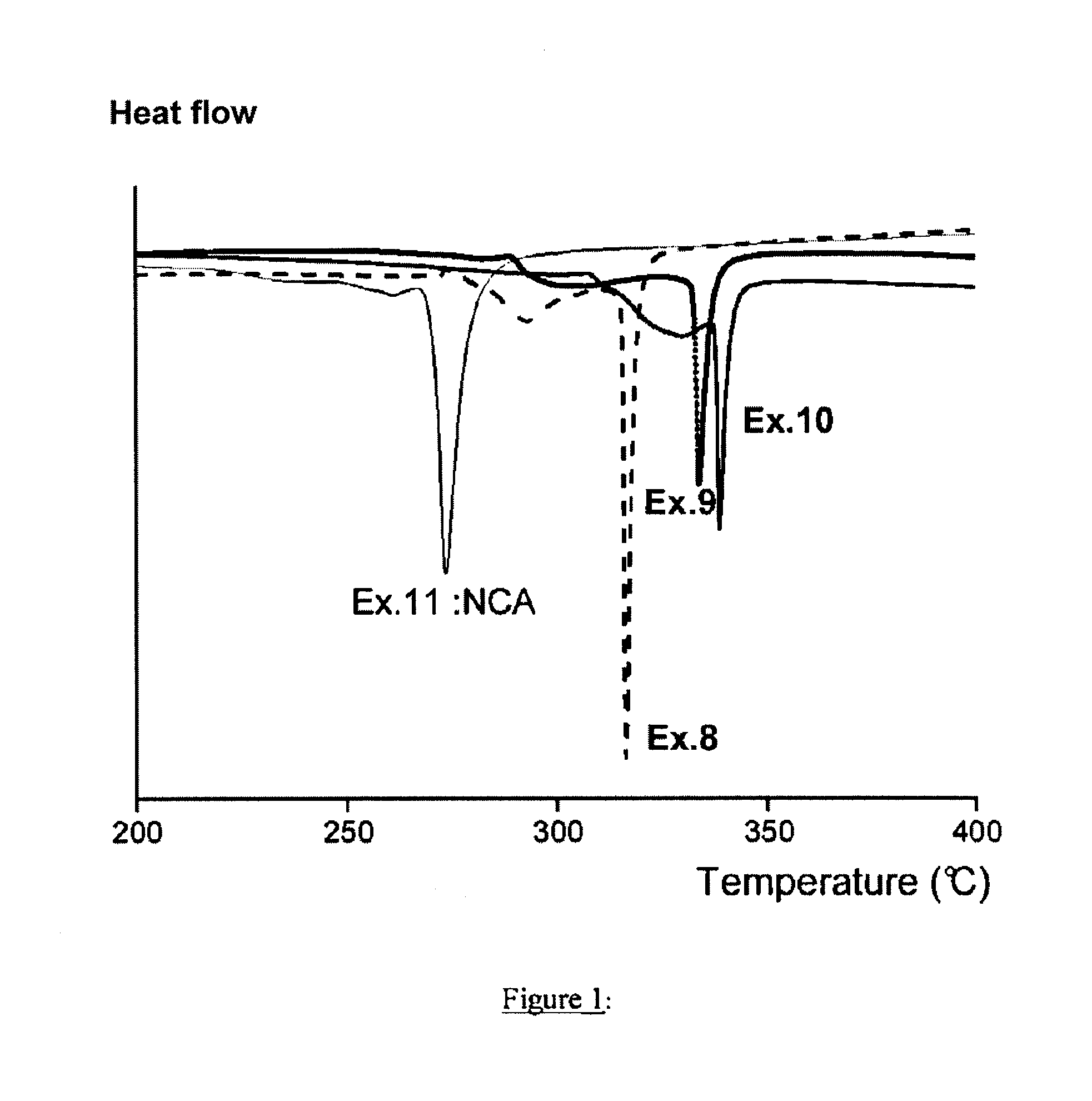
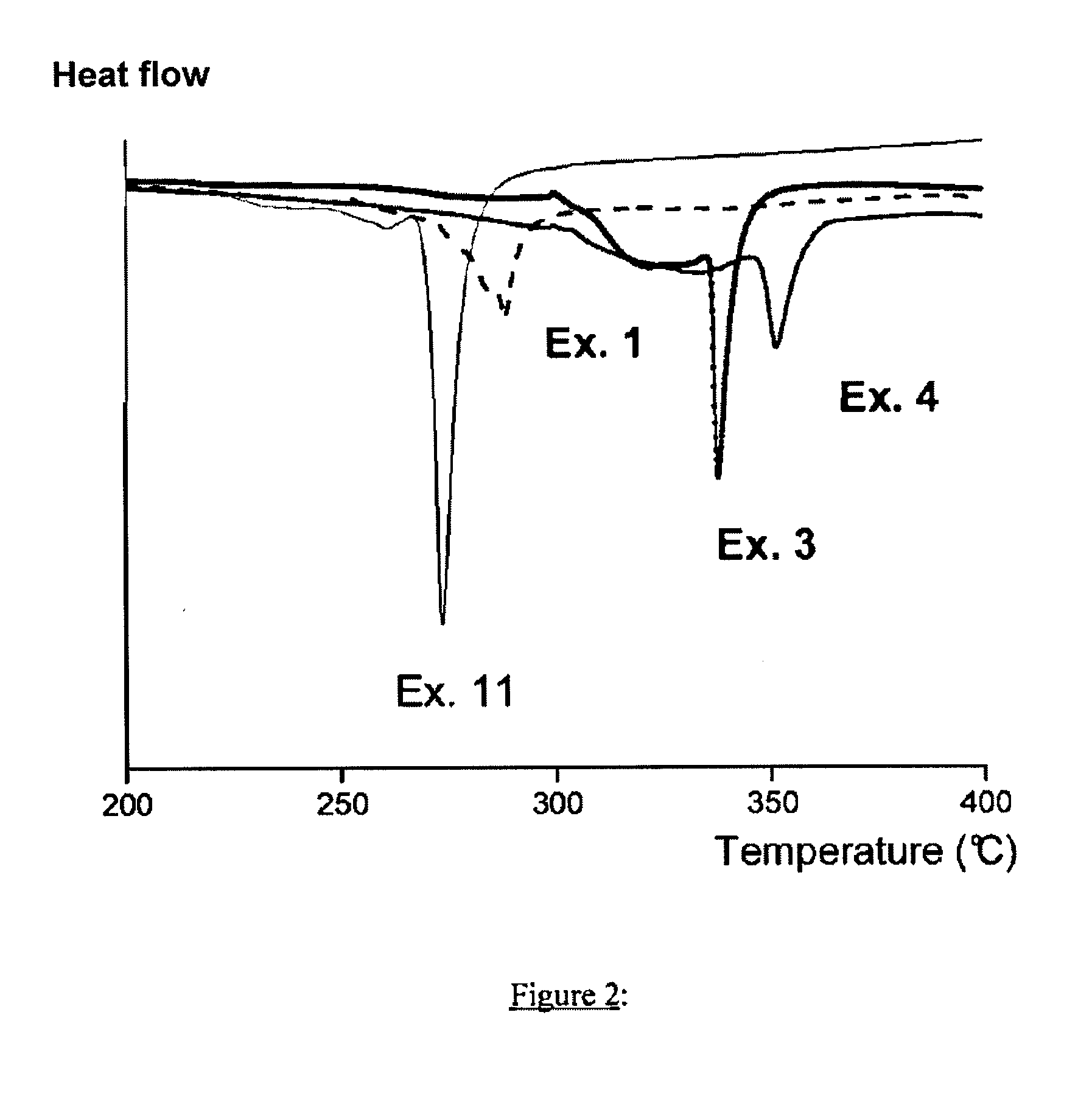
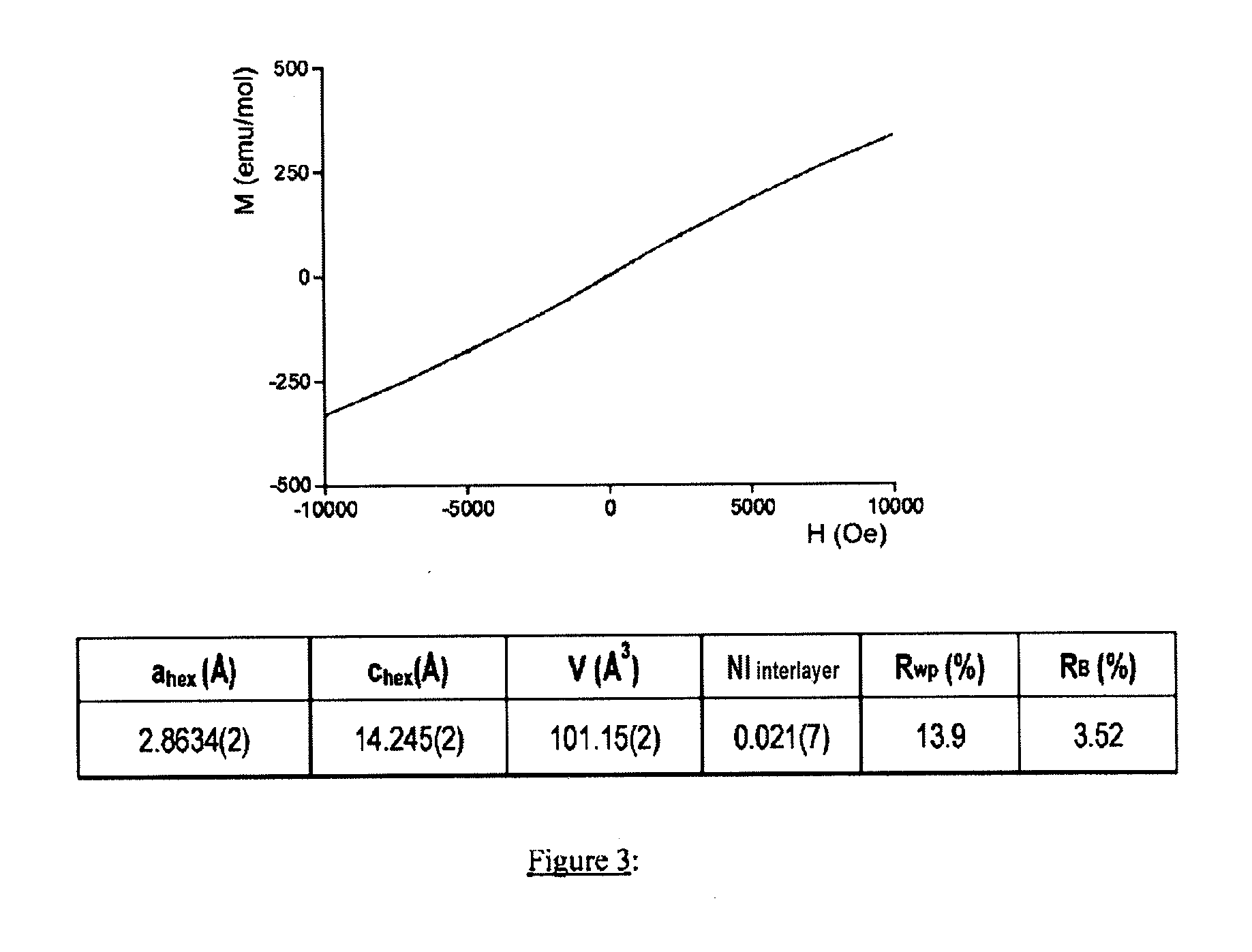
[0065]Improvement of the heat stability of the compounds according to the invention was able to be demonstrated by means of the Dif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| heat stability | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| heat instability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com