Chromatography medium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
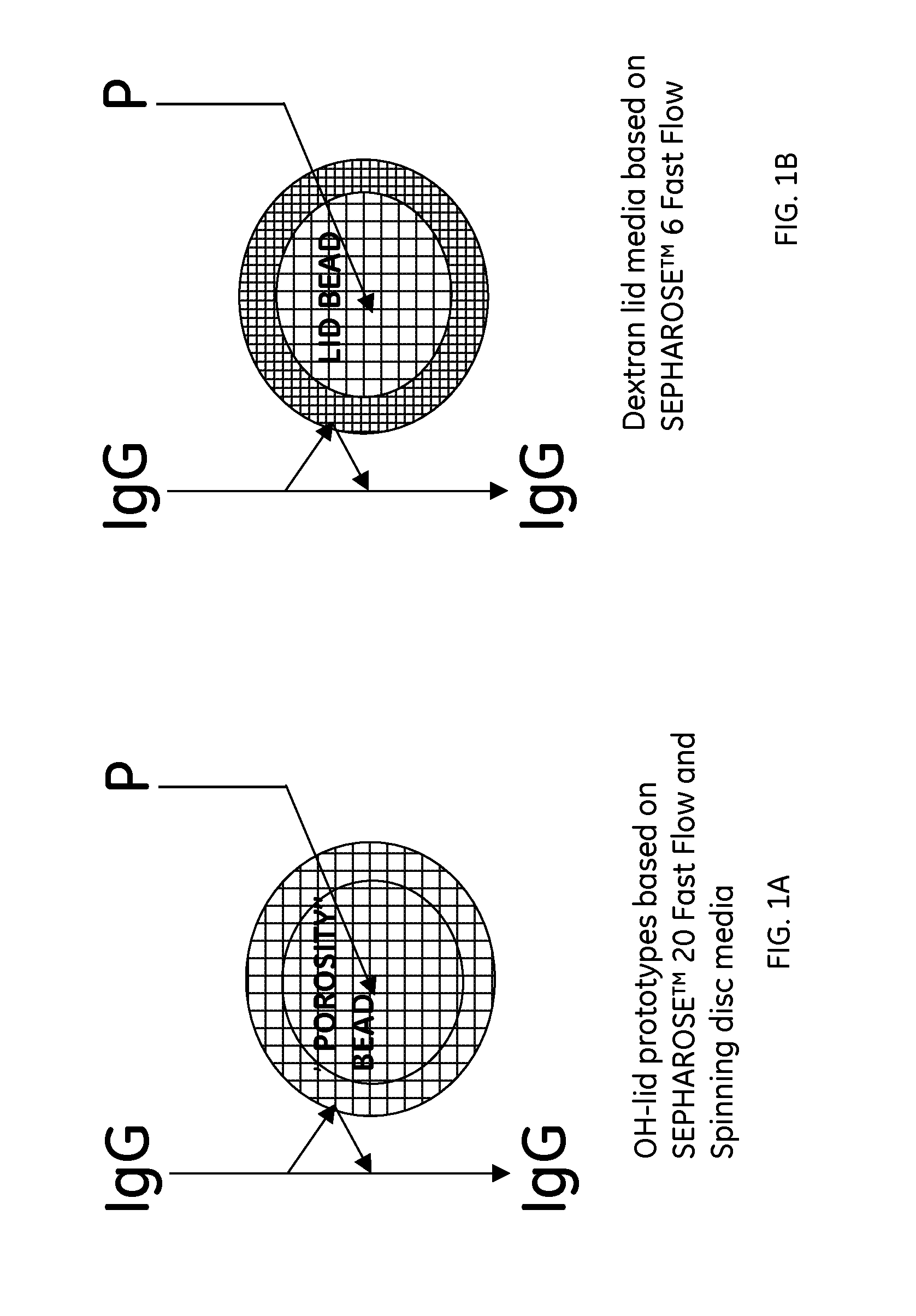
Preparation of Octyl Media Based on SEPHAROSE™ 20 Fast Flow (A), SEPHAROSE™ 6 Fast Flow (B), and Spinning Disc Media (C)
[0053]Volumes of matrix refer to settled bed volume and weights of matrix given in gram refer to suction dry weight. For large scale reaction stirring is referring to a suspended, motor-driven stirrer since the use of magnet bar stirrer is prompt to damage the beads. Small-scale reactions (up to 20 mL of gel) were performed in closed vials and stirring refers to the use of a shaking table.
[0054]Conventional methods were used for the analysis of the functionality and the determination of the degree of allylation, epoxidation, or the degree of substitution of ion exchanger groups on the beads.
A: Preparation of Lid-OH core-octyl SEPHAROSE™ 20 Fast Flow
A1: Preparation of SEPHAROSE™ 20 Fast Flow
[0055]Agarose (50 g) was dissolved in water (250 g) by heating at 95° C. for approximately 10 hours. The solution was added to toluene (375 mL) and ethyl cellulose (35 g) in an e...
example 2
Chromatographic Evaluation of the Three Prototypes Based on Octyl Ligands in the Core of the Beads
[0074]The three different octyl media to be investigated (Prototypes: lid-OH core-octyl SEPHAROSE™ 20 Fast Flow, lid-OH core-octyl spinning disc and lid-dextran octyl core SEPHAROSE™ Fast Flow), with respect to breakthrough capacity, were packed in HR 5 / 5 columns and the sample solution was pumped at a flow rate of 0.3 or 1.0 mL / min through the column after equilibration with buffer solution. The breakthrough capacity was evaluated at 10% of the maximum UV detector signal (280 nm). The maximum UV signal was estimated by pumping the test solution directly into the detector. The breakthrough capacity at 10% of absorbance maximum (Qb10%) was calculated according to the formula:
Qb10%=(TR10%−TRD)×C / Vc
where TR10% is the retention time (min) at 10% of absorbance maximum, TRD the void volume time in the system (min), C the concentration of the sample (4 mg protein / mL) and VC the column volume ...
example 3
Purification of Influenza Virus
[0125]When producing influenza virus at large scale aiming at influenza vaccines it is important to reduce the levels of protein and DNA in the final preparation.
[0126]The particles of the present invention are well suited for the purification of viruses since viral particles are significantly larger in size than most of the contaminants.
[0127]This is illustrated in the following example.
Analysis Methods
Virus Concentration
[0128]The DotBlot HA assay was used according to a standard protocol.
Dna Concentration
[0129]The PICOGREEN® DNA assay was used according to the manufacturers instruction (available from Invitrogen).
Protein Concentration
[0130]The Bradford protein assay was used according to the manufacturers instruction. (Available from Bio-Rad)
Agarose Gel Electrophoresis for Analysis of Molecular Weight Distribution in DNA Sample.
[0131]An E-GEL® Agarose Gel 0.8% (Invitrogen) precast gel was used according to the manufacturers instructions
[0132]The DNA ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Ionic strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com