Methods and compositions for sustained delivery of drugs
a topical delivery and composition technology, applied in drug compositions, metabolic disorders, cardiovascular disorders, etc., can solve the problems of limited drug delivery system, unique and fairly challenging hurdles for drug delivery, limited transdermal systemic delivery of drugs, etc., to achieve the effect of enhancing uptake and maintaining the health of ideas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Treatment of Severe Dry Eye
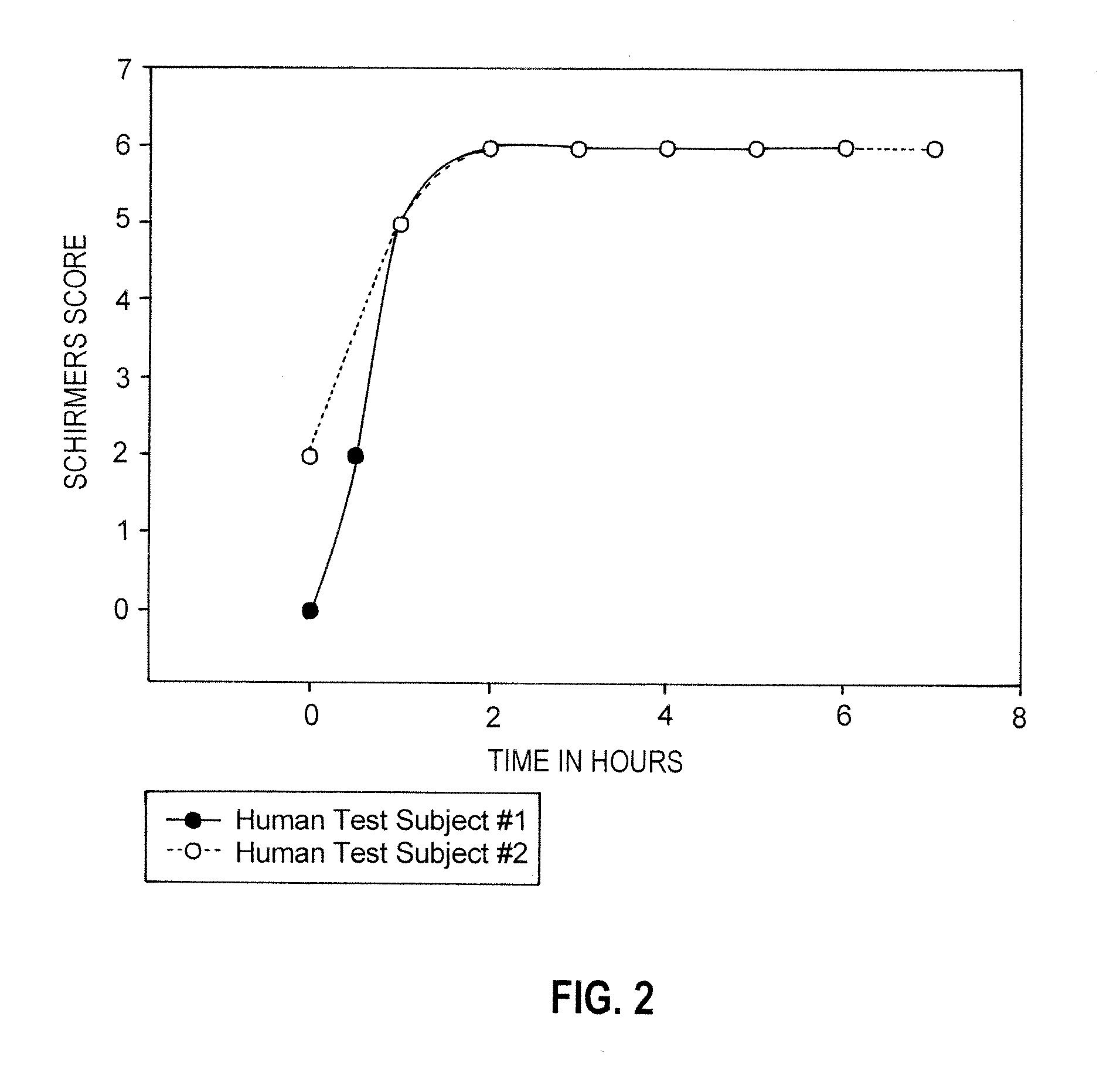
[0112]Two human patients with preexisting symptomatic severe dry eye were treated as follows. The dry eye condition was assessed by the Schirmers tear test and corneal observations. Each subject received a single application of a lotion containing 1% of pilocarpine and 0.16% caffeine applied to the upper eyelid of the more severely affected eye by direct application of the lotion to the upper eyelid. The effect of treatment was then measured over six hours in regards to tear production and comfort level of the patient.
[0113]There was a rapid increase in tear production in both affected eyes in both subjects. There was an exponential increase in tear production in both patients within one hour of application that was sustained for six hours, as can be seen in FIG. 2.
[0114]Prior to treatment both patients ranked their comfort level of the affected eye as 0 on a scale of 0 to 4 with 0 being the most irritated and 4 being the most comfortable. After applicatio...
example 2
[0115]A normal human subject was selected to determine the effects of a single application of percutaneous upper eyelid application of a cream / lotion containing 0.2% timolol and 0.16% caffeine to the outer surface skin of one upper eyelid. The intraocular pressure was recorded in both the treated and untreated eye both before and after application of the composition. Additionally, the subject's vital signs, including pulse rate and blood pressure were recorded at the same time intervals as eye pressure was recorded. The effect of treatment was measured for over fifty two hours.
[0116]After a single application of timolol to one eyelid there was a rapid decline in intraocular pressure in both eyes as shown in FIG. 4. The treated eye showed a 73% drop in intraocular pressure within four hours of treatment, which returned to baseline eye pressure within 52 hours. The untreated eye rapidly dropped intraocular pressure by 50% within the same time frame as the treated e...
example 3
Effects of Topical Application of Physostigmine to One Eyelid
[0118]A normal human subject was selected to determine the effects of a single application of percutaneous upper eyelid application of a cream / lotion containing 5% physostigmine, a parasympathetic agonist, 0.16% caffeine to the outer surface skin of one upper eyelid. The effects on tear production, intraocular pressure and pupilary constriction were measured in both the treated and untreated eye both before and after application of the composition. Additionally, the subject's vital signs, including pulse rate and blood pressure were recorded at the same time intervals as eye pressure was recorded. The effect of treatment was measured for over twelve hours. Prior to treatment, the treated eye showed a dryness level of 0, while the untreated eye had a dryness level of 2 mm by Schirmers measurement.
[0119]After a single application of physostigmine to one eyelid there was a rapid increase in tear production from 0 to 5 mm in t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wt % | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com