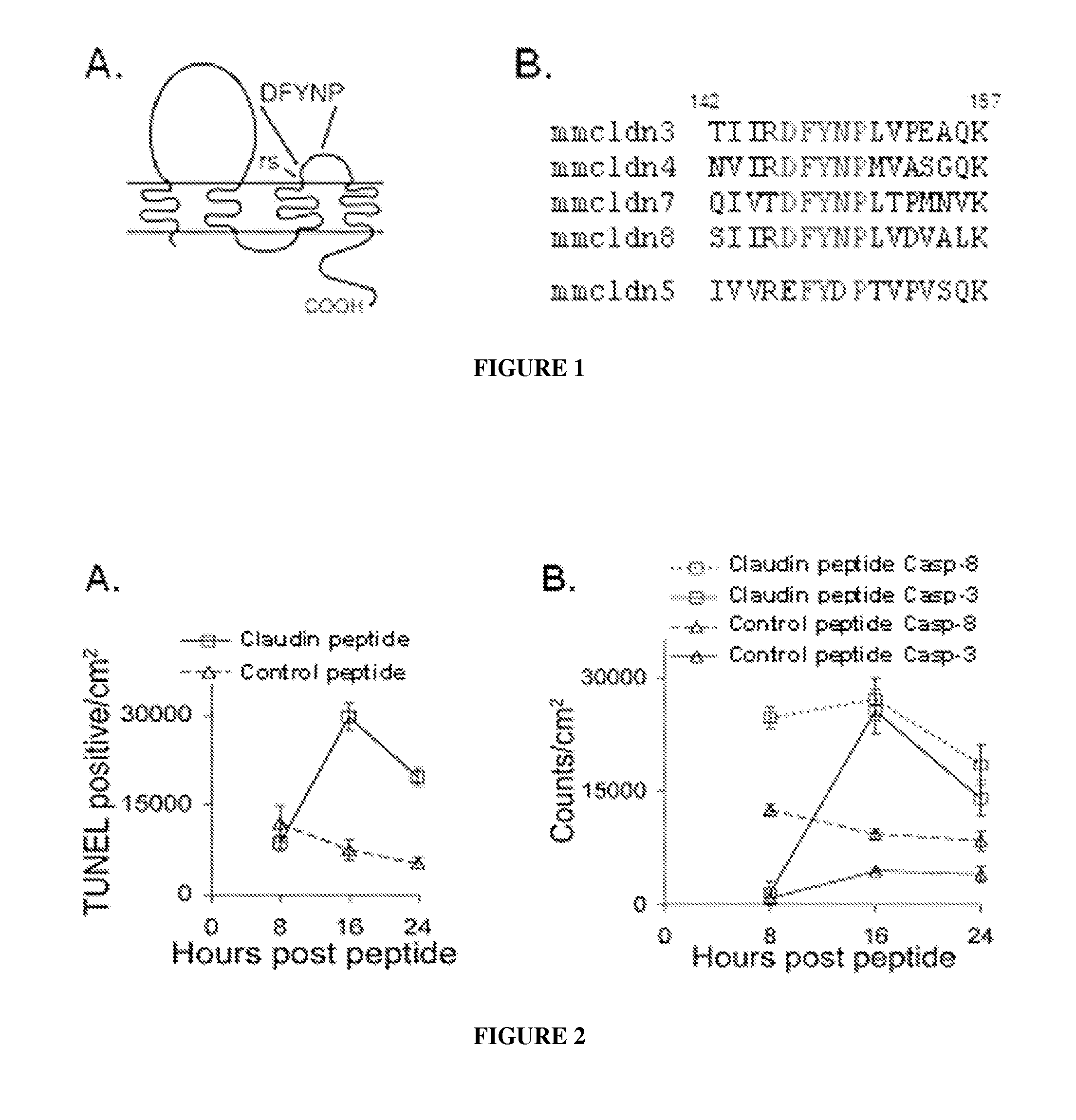
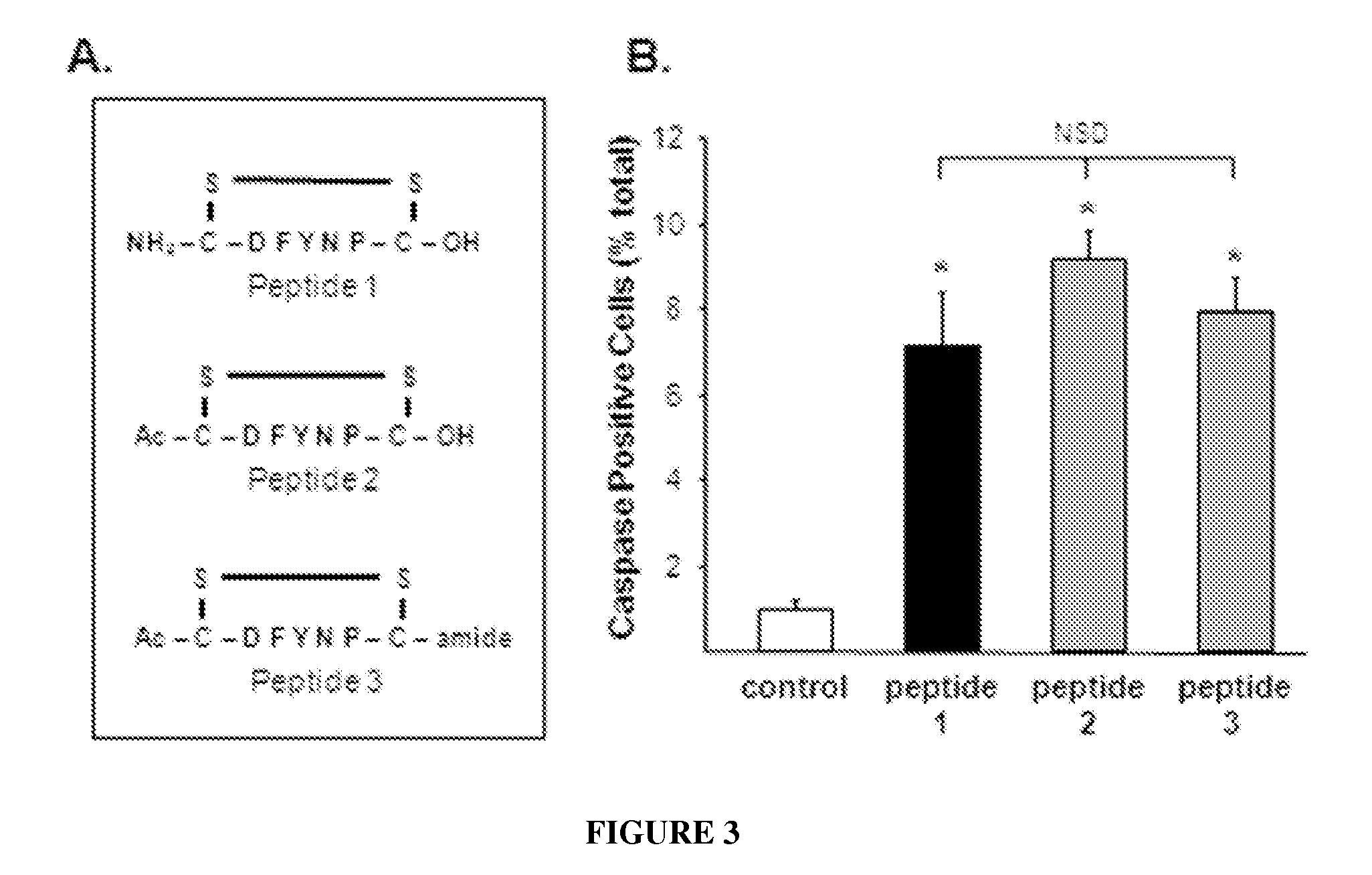
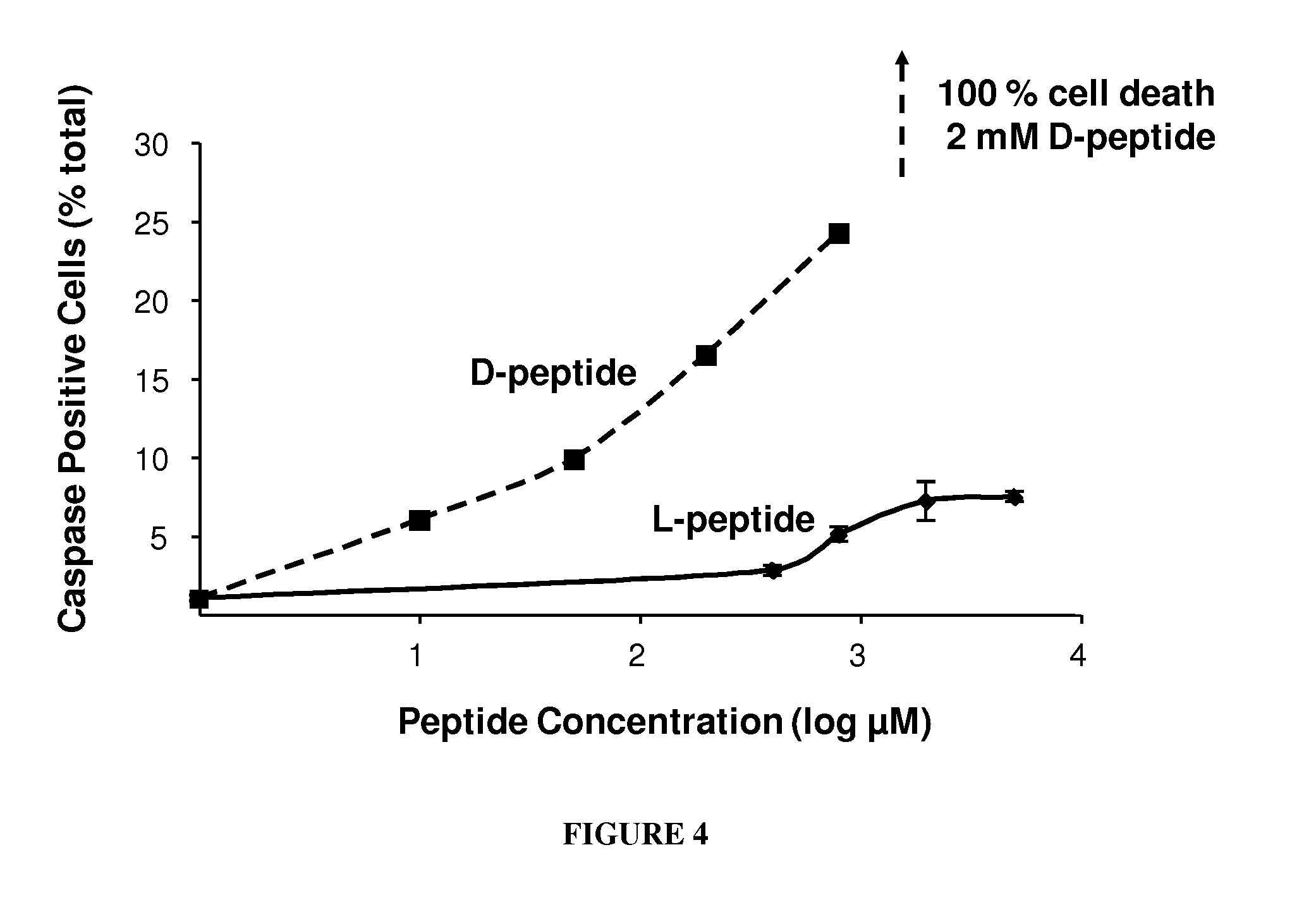
Tight junction protein modulators and uses thereof
a protein modulator and tight junction technology, applied in the direction of peptides, drug compositions, peptides/protein ingredients, etc., can solve the problems of increasing the tumorigenicity of cells transplanted into animals, affecting the survival rate of animals, and increasing the tumorigenicity of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Materials and Methods
[0085]
ABBREVIATIONNAMEFmocFluorenylmethoxycarbonylHOBt1-Hydroxybenzotriazole monohydrateDICDiisopropylcarbodiimideDMFN,N-DimethylformamideTFATrifluoroacetic acidTrtTrityltBut-ButylOtBut-Butoxy
Antibodies:
[0086]Various antibodies were obtained as follows: Anti occludin, Zymed® clone OCOC-3F10; anti Fas, BD Biosciences clone 13; anti ZO-1, Chemicon® MAB 1520; anti FADD, USBiological clone 12E7; anti MUC1, Abcam Inc. clone EP1024Y; anti-caspase-3, Cell Signaling Technology® (8G10); anti-caspase-8, Axxora® (1G12).
Cells and Cell Culture:
[0087]CIT3 mouse mammary epithelial cells were grown as described by Toddywalla V S et al., J. Pharmacol. Exp. Ther., 1997, 280, 669-676. To differentiate cells, this growth medium was modified by removal of EGF and addition of 3 μg / ml each of ovine prolactin and hydrocortisone (differentiation media).
[0088]EPH4 mouse mammary epithelial cells were grown as described by Reichmann E et al., J. Cell Biol., 1989, 108, 1127-1138. To differe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com