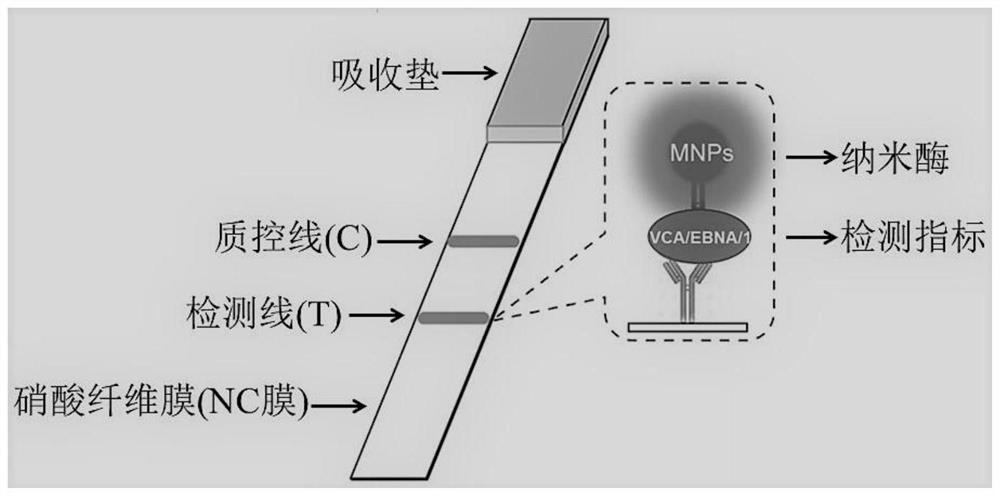
Test strip for detecting cerebral hemorrhage 31-kDa Occludin after thrombolysis as well as preparation method and application of test strip
A 31-kdaoccludin and test strip technology, which is applied to measurement devices, instruments, disease diagnosis, etc., can solve the problem that the detection method of nano-enzyme detection test strips does not have universal applicability, The problem of low content in serum has achieved the effect of good market application value, good detection specificity and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Synthesis and Preparation of Anti-31-kDa Occludin Protein Antibody-Nanozyme Conjugate Complex
[0027] 1. Fe 3 o 4 Synthesis of nanozymes
[0028] Nanozyme was synthesized by hydrothermal method: Dissolve 0.3g FeCl3·6H2O in 20mL ethylene glycol, stir to dissolve, then add 1.5g sodium acetate, after the mixture is completely dissolved, seal it in an autoclave, and react at 200°C for 14 hour, the product obtained was magnetically separated, the supernatant was discarded, the precipitate was washed 3 times with ethanol, and then dried and stored at 50-60°C in an electric constant temperature drying oven to obtain Fe 3 o 4 nanozyme.
[0029] 34 Synthesis of Nanozyme-Anti-31-kDa Occludin Antibody Conjugate Complex
[0030] (1) Prepare 1 mL of activator, which is a mixture of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide EDC and N-hydroxysuccinimide NHS, and the EDC The ratio of the amount of substance to the NHS is 1:1;
[0031] (2) Add the Fe prepared by t...
Embodiment 2
[0034] Example 2 Preparation of Detecting 31-kDa Occludin Protein Nanozyme Test Strip
[0035] The preparation for detecting the 31-kDa Occludin protein nanozyme test strip comprises the following steps:
[0036] (1) Sample pad pretreatment: Soak the glass fiber membrane in the sample pad buffer solution to completely soak it, soak it for 5 minutes, take it out, drain and dry it for later use; the sample pad buffer solution is 1% BSA-PBS, 1% Tween- 20. The preparation method of the 1% BSA-PBS is as follows: 1 g of bovine serum albumin BSA is dissolved in 100 mL of phosphate buffer solution PBS, the pH of the phosphate buffer solution is 7.4, and the molar concentration of the phosphate buffer solution is It is 10mmol / L.
[0037] (2) Pretreatment of the reaction pad: soak another piece of glass fiber membrane in the buffer of the reaction pad until it is completely soaked; soak for 5 minutes, take it out and dry it and dry it for later use; the buffer of the sample pad is 1% B...
Embodiment 3
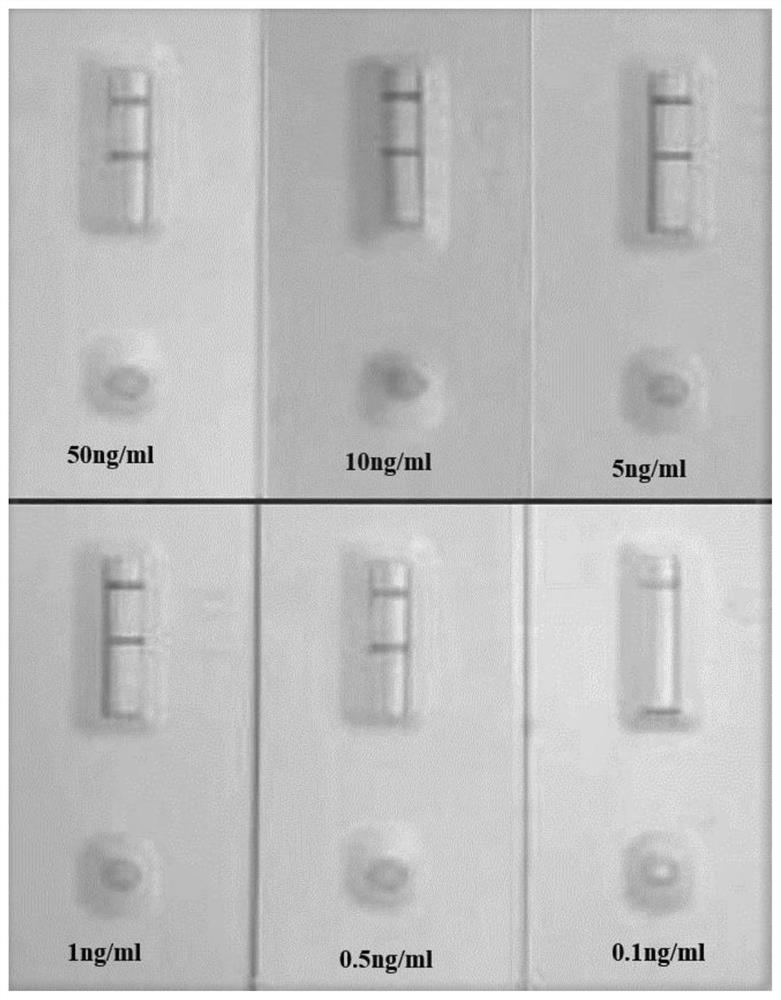
[0041] Example 3 Optimization of preparation process conditions for detecting 31-kDa Occludin protein nanozyme test strips
[0042] 1. Exploration of the optimal surface active type and concentration in the reaction pad buffer
[0043] (1) Preparation of different types and concentrations of reaction pad buffers and reaction pad pretreatment
[0044] A suitable surfactant can often enhance the reaction signal and eliminate false positives during the entire reaction process. On the contrary, if the surface activity is not suitable, it will often lead to problems such as confusion of test results and inability to distinguish between positive and negative. Therefore, adding a suitable surfactant to the reaction pad is very important for the detection performance of the 31-kDa Occludin protein nanozyme test strip. This experiment mainly explores the surfactant suitable for the detection of 31-kDa Occludin protein nanozyme. %, 0.75%, and 1% concentration gradients were added to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com