Nanocarrier compositions with uncoupled adjuvant
a technology of adjuvant and composition, applied in the direction of powder delivery, macromolecular non-active ingredients, inorganic non-active ingredients, etc., can solve the problem of not yet known optimal approach for augmenting the immune response with adjuvan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
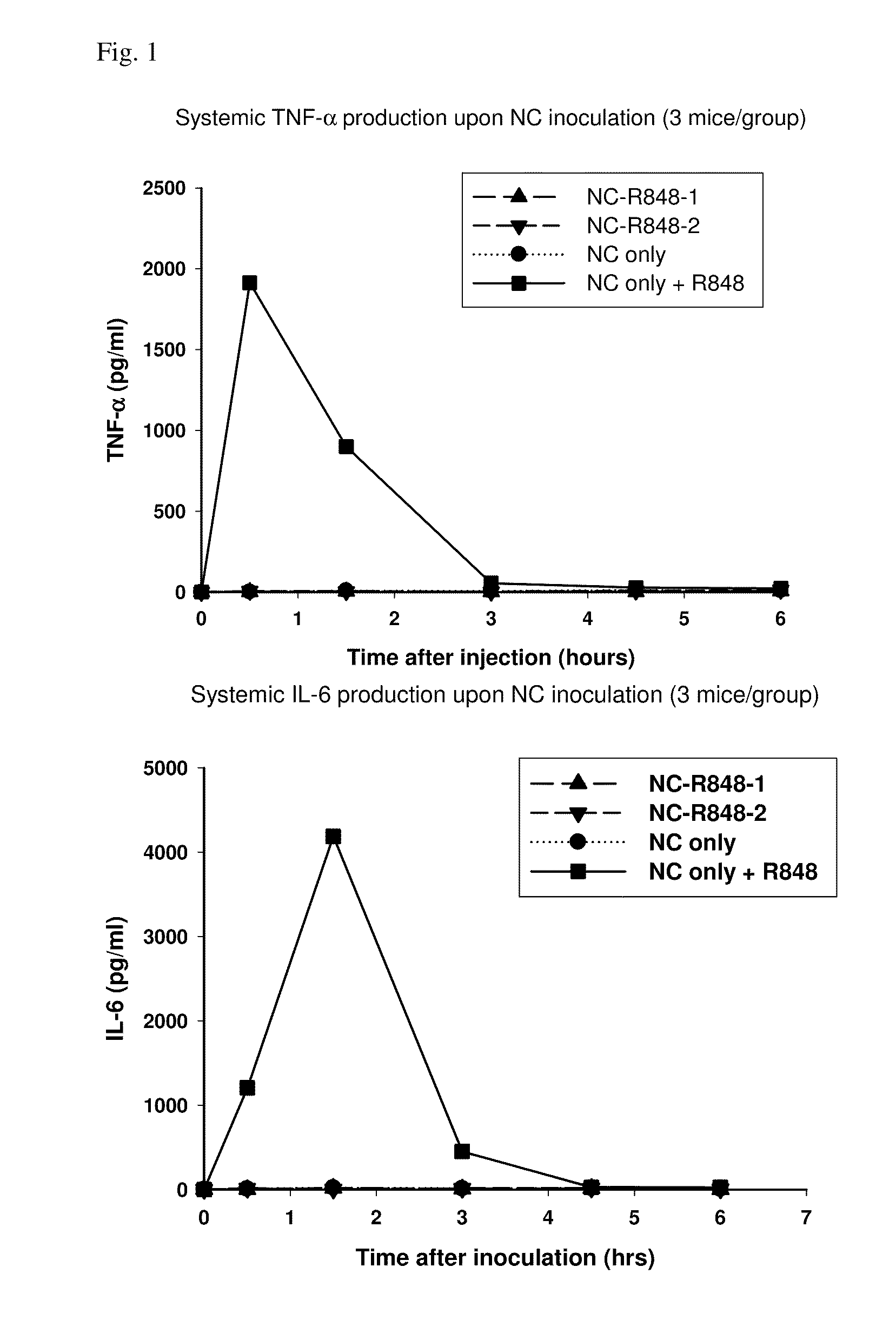
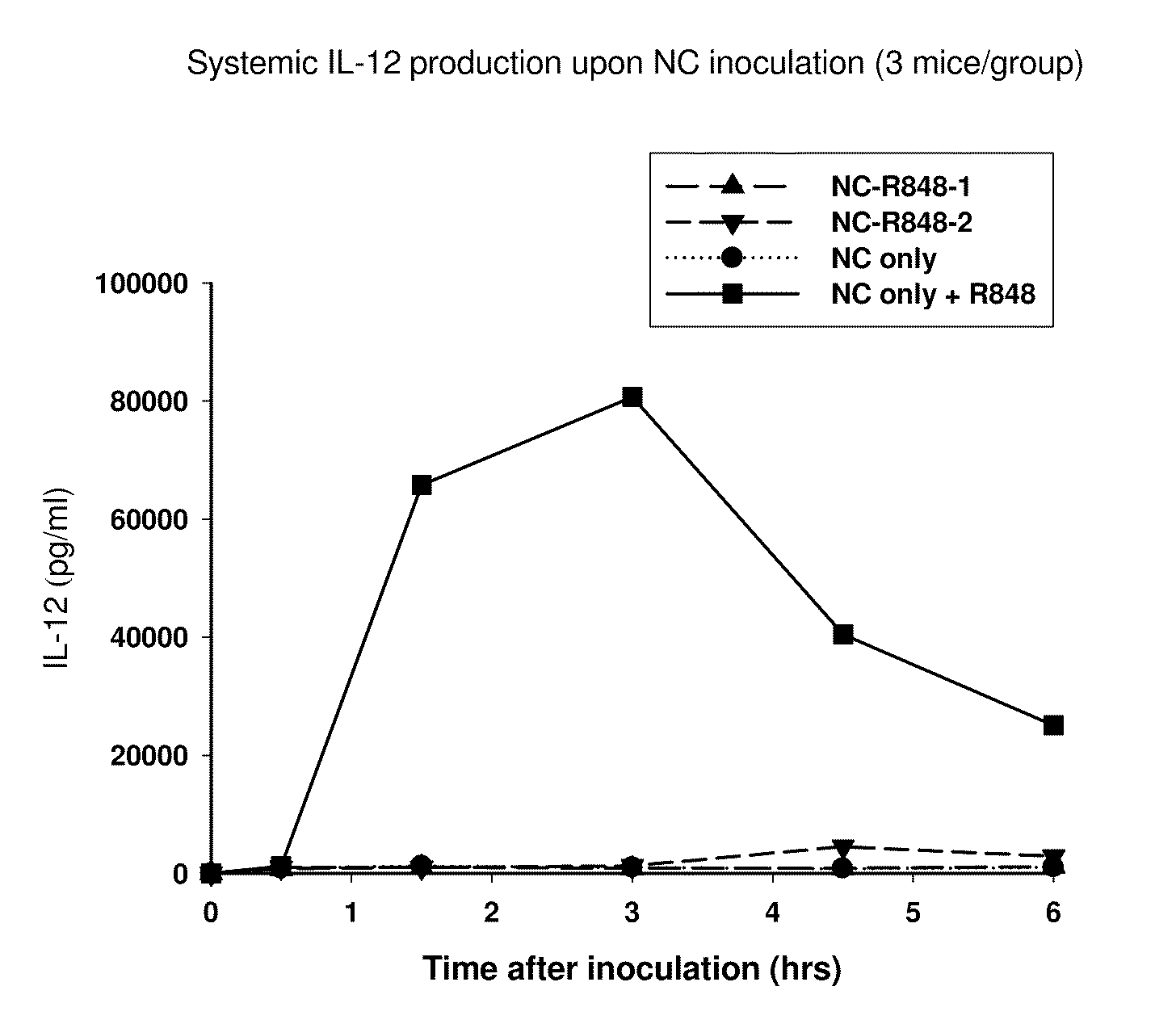
Administration of Nanocarrier and Admixed R848 Adjuvant Results in Strong Systemic Production of Inflammatory Cytokines
Materials for NC-R848-1 Nanocarrier Formulations
[0146]Ovalbumin peptide 323-339 amide acetate salt, was purchased from Bachem Americas Inc. (3132 Kashiwa Street, Torrance Calif. 90505. Part #4065609.) PLGA-R848 conjugate of 75 / 25 lactide / glycolide monomer composition and of approximately 4100 Da molecular weight having 5.2% w / w R848 content was synthesized. PLA-PEG-Nicotine with a nicotine-terminated PEG block of approximately 3,500 Da and DL-PLA block of approximately 15,000 Da was synthesized Polyvinyl alcohol (Mw=11,000-31,000, 87-89% hydrolyzed) was purchased from J.T. Baker (Part Number U232-08).
Methods for NC-R848-1 Nanocarrier Production
[0147]Solutions were prepared as follows:
[0148]Solution 1: Ovalbumin peptide 323-339 @ 70 mg / mL was prepared in 0.13N hydrochloric acid at room temperature.
[0149]Solution 2: PLGA-R848 @ 75 mg / mL and PLA-PEG-Nicotine @ 25 mg / mL...
example 2
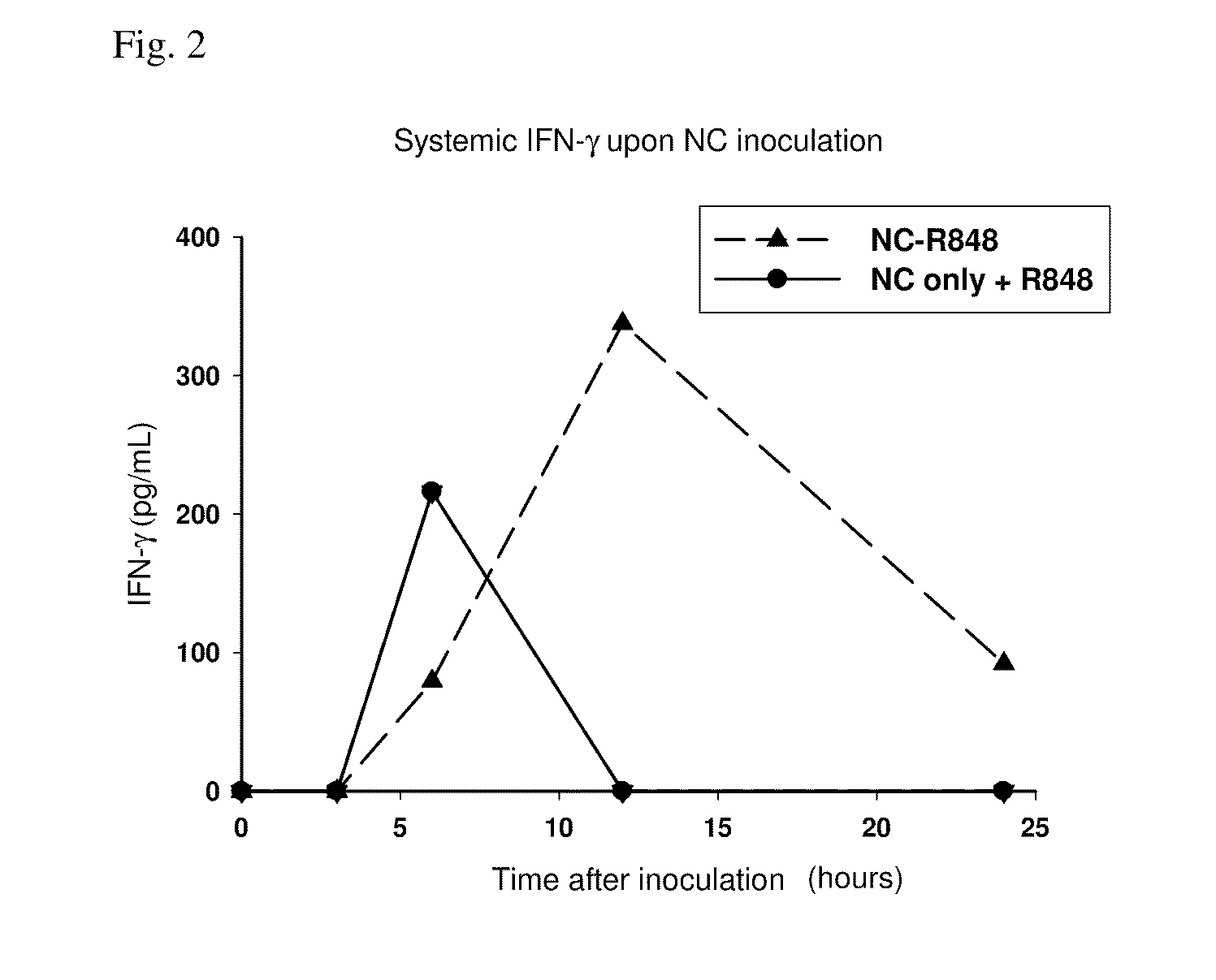
Coupling of Nanocarrier to R848 Adjuvant does not Inhibit Systemic Production of Immune Cytokine IFN-γ
Materials for NC-R848 Nanocarrier Formulations
[0168]Ovalbumin peptide 323-339 amide acetate salt was purchased from Bachem Americas Inc. (3132 Kashiwa Street, Torrance Calif. 90505. Part #4065609.) PLGA-R848 conjugate of 75 / 25 lactide / glycolide monomer composition and of approximately 4100 Da molecular weight having 5.2% w / w R848 content was synthesized. PLA-PEG-Nicotine with a nicotine-terminated PEG block of approximately 3,500 Da and DL-PLA block of approximately 15,000 Da was synthesized. Polyvinyl alcohol (Mw=11,000-31,000, 87-89% hydrolyzed) purchased from J.T. Baker (Part Number U232-08).
Methods for NC-R848 Nanocarrier Production
[0169]Solutions were prepared as follows:
[0170]Solution 1: Ovalbumin peptide 323-339 @ 70 mg / mL was prepared in 0.13N hydrochloric acid at room temperature.
[0171]Solution 2: PLGA-R848 @ 75 mg / mL and PLA-PEG-Nicotine @ 25 mg / mL in dichloromethane was p...
example 3
Addition of Free Adjuvant Augments Immune Response
Materials for NC-Nic w / o R848 Nanocarrier Formulations
[0183]Ovalbumin peptide 323-339 amide TFA salt was purchased from Bachem Americas Inc. (3132 Kashiwa Street, Torrance Calif. 90505. Part #4064565.) PLA with an inherent viscosity of 0.19 dL / g was purchased from Boehringer Ingelheim (Ingelheim Germany. Product Code R202H). PLA-PEG-Nicotine with a nicotine-terminated PEG block of approximately 3,500 Da and DL-PLA block of approximately 15,000 Da was synthesized. Polyvinyl alcohol (Mw=11,000-31,000, 87-89% hydrolyzed) was purchased from J.T. Baker (Part Number U232-08).
Methods for NC-Nic w / o R848 Nanocarrier Production
[0184]Solutions were prepared as follows:
[0185]Solution 1: Ovalbumin peptide 323-339 @ 69 mg / mL was prepared in 0.13N hydrochloric acid at room temperature.
[0186]Solution 2: PLA @ 75 mg / mL and PLA-PEG-Nicotine @ 25 mg / mL in dichloromethane was prepared by dissolving PLA @ 100 mg / mL in dichloromethane and PLA-PEG-Nicotin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com