Conserved Hemagglutinin Epitope, Antibodies to the Epitope and Methods of Use
a technology of hemagglutinin and epitope, applied in the field of influenza, can solve the problems of human health threats and inability to always be fully successful, and achieve the effect of preventing or reducing the severity of an influenza infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A. Example 1
Isolation and Analysis of Neutralizing Antibodies to Hemagglutinin Stem Region
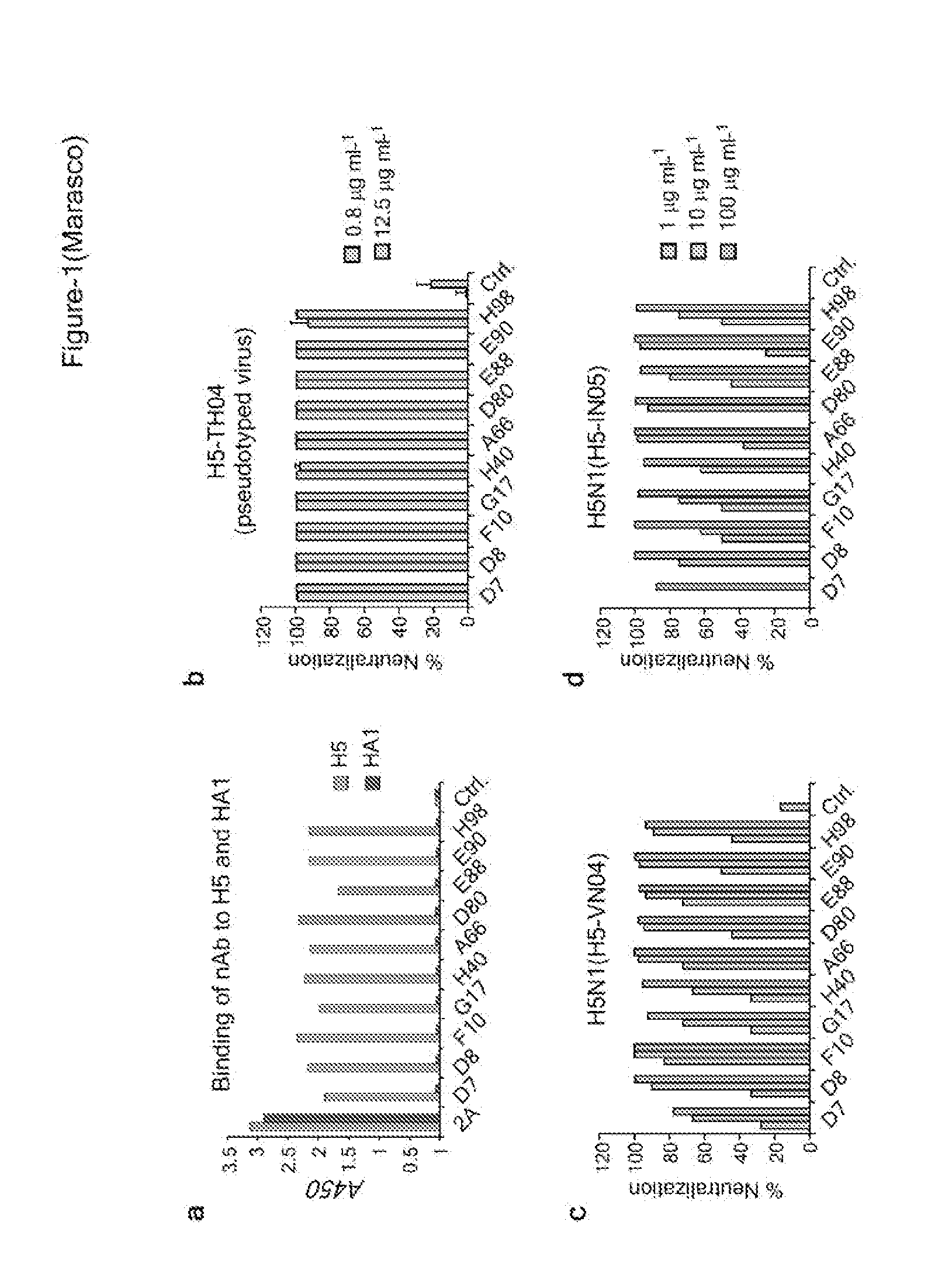
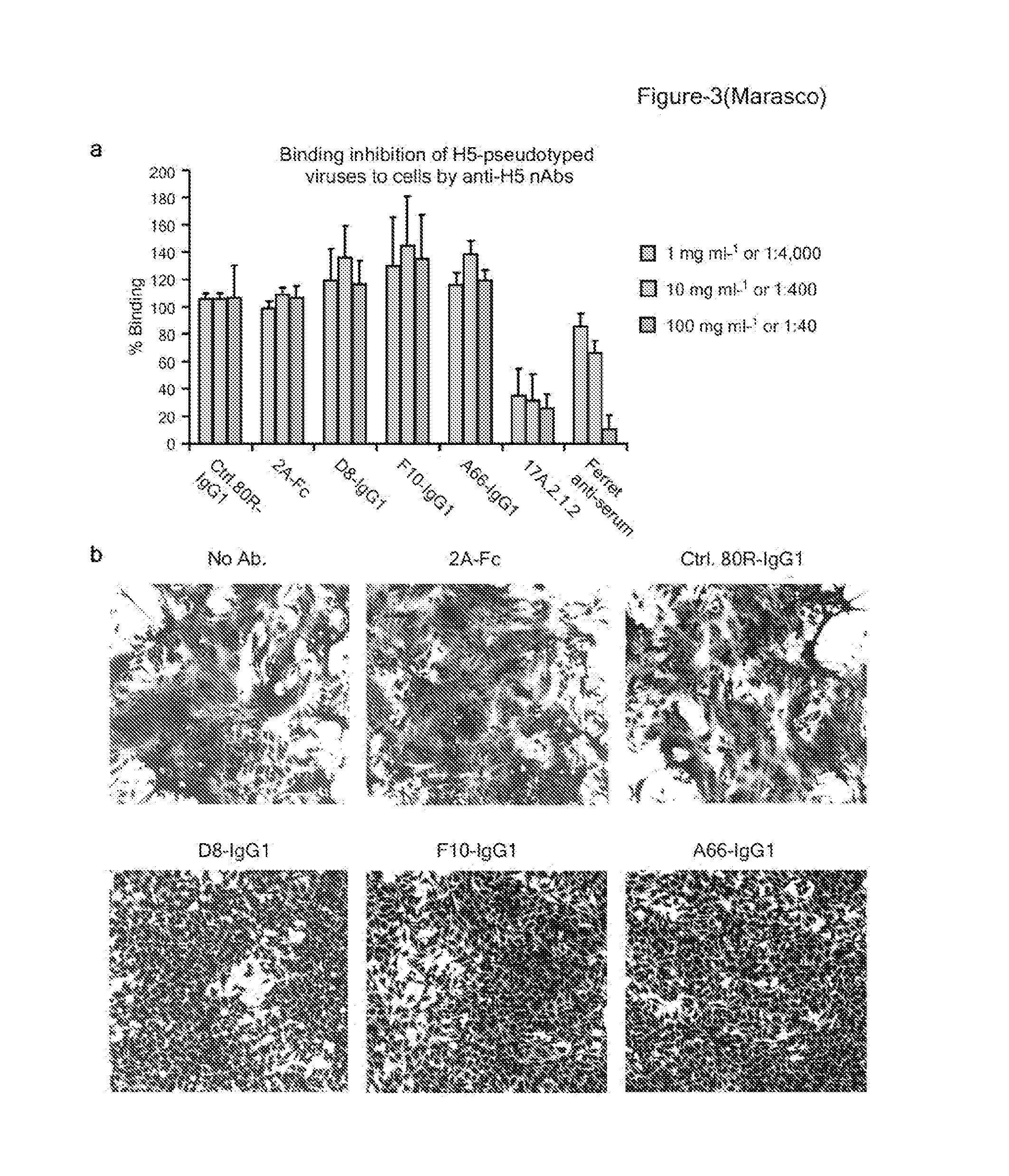
[0427]In this example, a phage-display antibody library and recombinant H5 trimeric ectodomain were used to isolate a group of high-affinity neutralizing mAbs (“nAbs”) that were potent inhibitors of H5N1 viral infection in vitro and in vivo. Based on crystallographic and functional studies, it was shown that the nAbs bind to a common epitope—a highly conserved pocket in the stem region of HA containing the “fusion peptide”—that rationalizes their ability to block membrane fusion rather than cell attachment. Sequence and structural analysis of all 16 HA subtypes points to the existence of just two variants of this epitope, corresponding to the two classic phylogenetic groupings of HA (Groups 1 and 2). Eight further Group 1 HA subtypes were tested and demonstrated a remarkable and unprecedented cross-subtype binding and / or neutralization spectrum. Since a Group 1 subtype (H5) was used for panning...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com