Senescence marker, method for evaluating senescence inhibitor, and cancer inhibitor
a senescence inhibitor and cancer technology, applied in the field of senescence markers, can solve the problems that the use of hsa-mir-22 for diseases such as cancer has not been elucidated in detail, and achieve the effect of inhibiting the growth, invasion and/or metastasis of cancer, and being easy to evalua
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
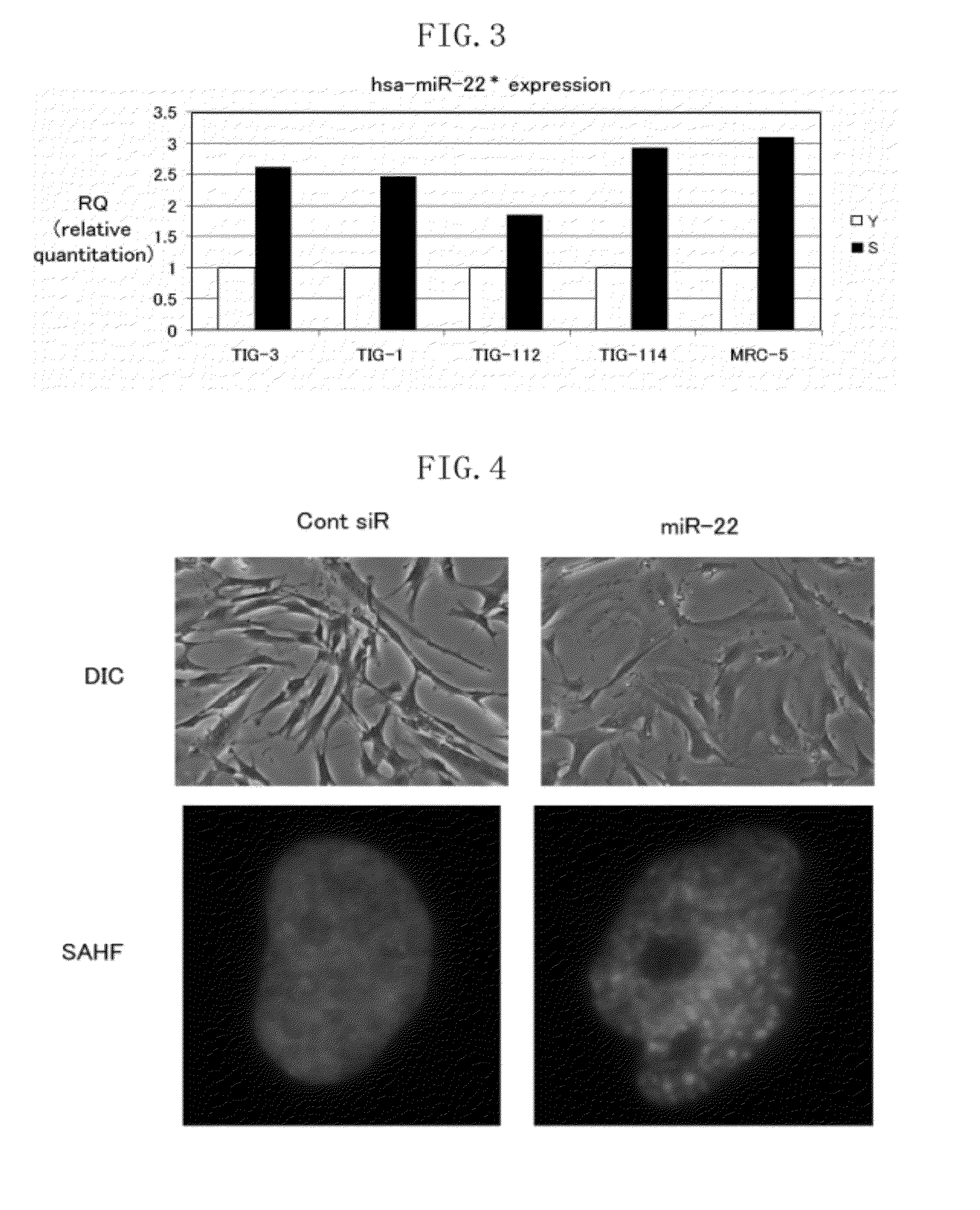
[0100]Example 1 describes an example in relation to the expression levels of hsa-miR-22* in young and senescent human fibroblasts. The present inventors investigated the expression levels of hsa-miR-22* in young human fibroblasts and senescent human fibroblasts (cells that experienced a larger number of times of division than young cells) by quantitative real-time RT-PCR. Details of the investigation are described below.
[0101]The expression level of hsa-miR-22* was investigated for human fibroblasts TIG-3 cells, TIG-1 cells, TIG-112 cells, TIG-114 cells and MRC-5 cells. The TIG-3 cells, TIG-1 cells, TIG-1 12 cells and TIG-114 cells were cultured using DMEM (Dulbecco's Modified Eagle's Medium) supplemented with 10% FBS (Fetal Bovine Serum) and an antibiotic. The MRC-5 cells were cultured in DMEM / F-12 medium (1:1, v / v) supplemented with SAP (0.2 mM serine, 0.1 mM aspartic acid, 1.0 mM pyruvate), 10% FBS and an antibiotic. All these cells were cultured using a humidified incubator at 3...
example 2
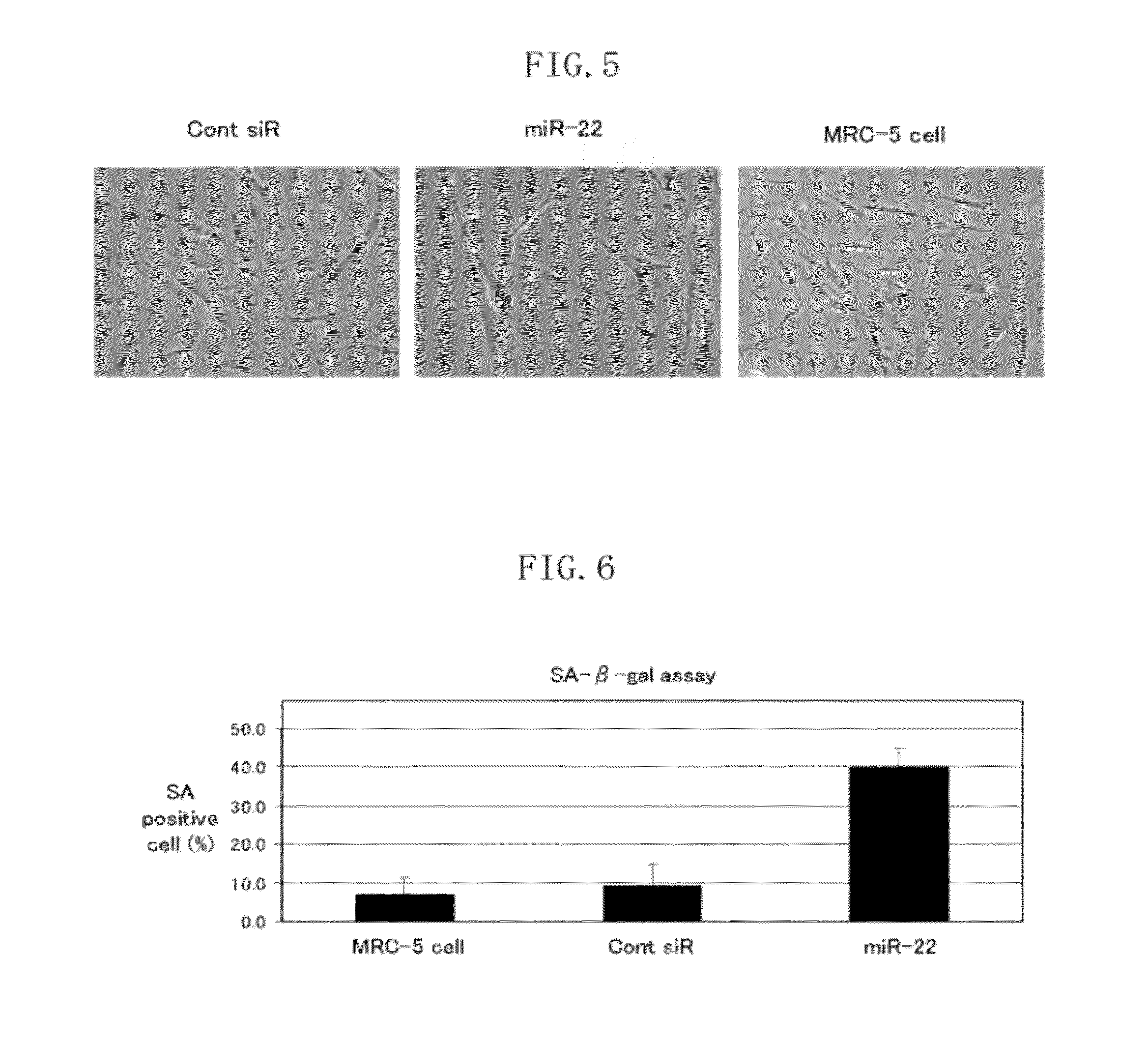
[0104]The present Example 2 describes an example in relation to cases where double-stranded hsa-miR-22 was introduced into human fibroblasts. The present inventors investigated whether or not phenomena found in senescent cells are observed when double-stranded hsa-miR-22 was introduced into human fibroblasts (MRC-5 cells). Details of the investigation are described below.
[0105]First, the present inventors investigated whether or not formation of senescence-associated heterochromatic foci (hereinafter referred to as SAHF), which is a marker for senescent cells, is observed in cases where double-stranded hsa-miR-22 was introduced into human fibroblasts (MRC-5 cells). According to the protocol for Lipofectamine RNAi Max (Invitrogen), 10 nM double-stranded hsa-miR-22 (B-bridge, this also applies to later-mentioned Examples) or AllStars Negative Control siRNA (Qiagen) was introduced into MRC-5 cells. The introduction efficiency was assumed to be not less than 90% based on measurement usi...
example 3
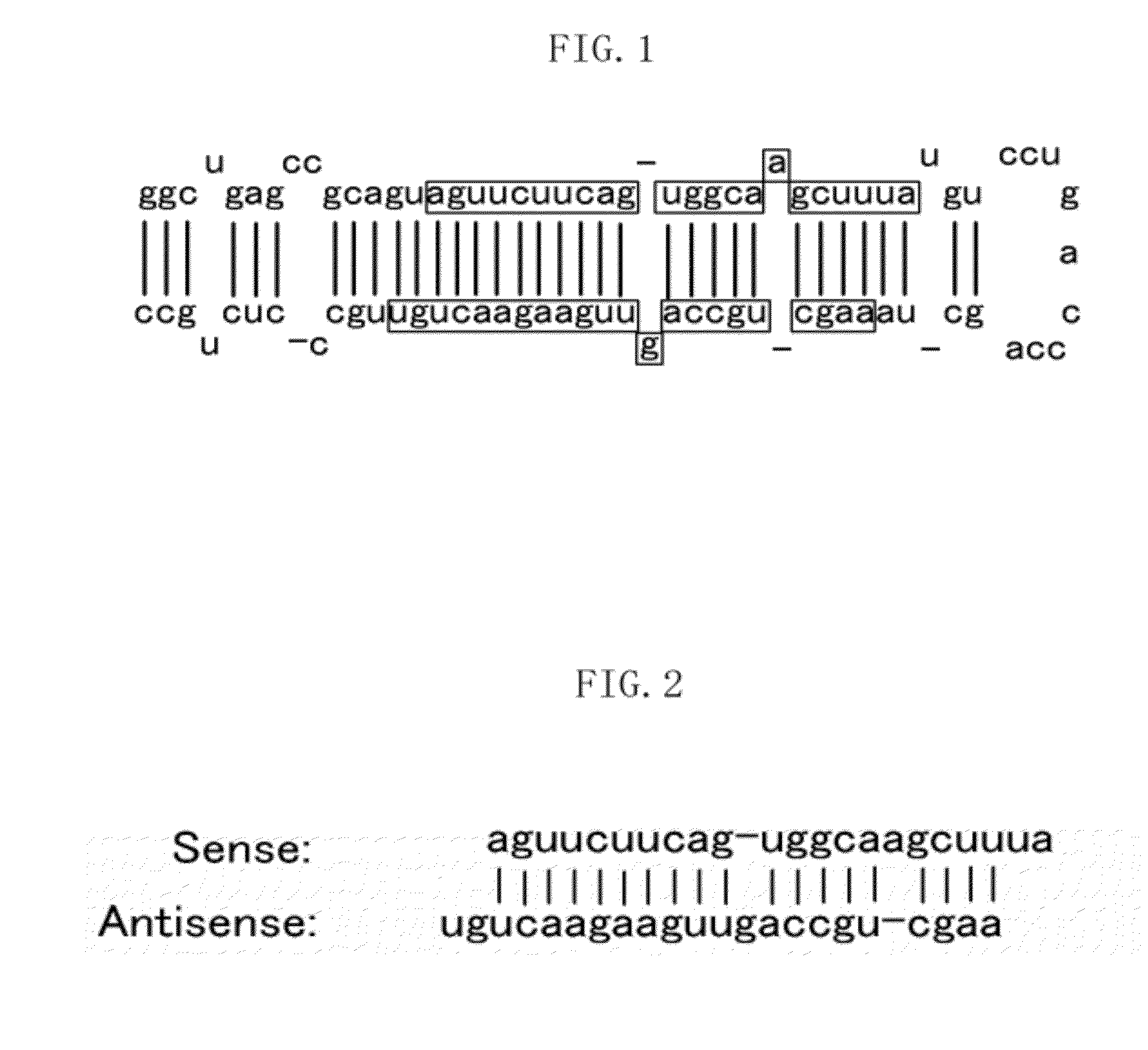
[0117]The present Example 3 describes an example in relation to cases where Pre-hsa-miR-22 (see FIG. 1) was introduced into human fibroblasts (MRC-5 cells) by lentivirus infection. The present inventors confirmed whether or not phenomena that specifically accompany cellular senescence (the SA-β-gal activity and inhibition of the cell growth) are observed when Pre-hsa-miR-22, which is a precursor of mature hsa-miR-22, was introduced into human fibroblasts.
[0118]First, Lipofectamine LTX Plus reagent (Invitrogen) was used to cotransfect 293T cells with 0.9 μg of Pre-hsa-miR-22 or a lentivirus vector as a control empty vector (these were manufactured by System Biosciences), and 0.9 μg of a packaging plasmid mix (pPACK-H1-GAG, pPACK-H1-Rev and pVSV-G), thereby producing a lentivirus. Forty-eight hours after the transfection, the supernatant obtained by filtration through a 0.45 μm filter was collected, and the collected supernatant was directly used for infection of MRC-5 cells. Although...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com