X-ray contrast media compositions and methods of using the same to treat, reduce or delay the onset of CNS inflammation and inflammation associated conditions
a technology of contrast media and compositions, applied in the direction of drug compositions, peptide/protein ingredients, biocides, etc., can solve the problems of more achieve the effects of improving the treatment of allergic and nasal inflammatory conditions, relieving not only nasal itch, secretions and sneezing, and improving the treatment of severe allergic and inflammatory reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
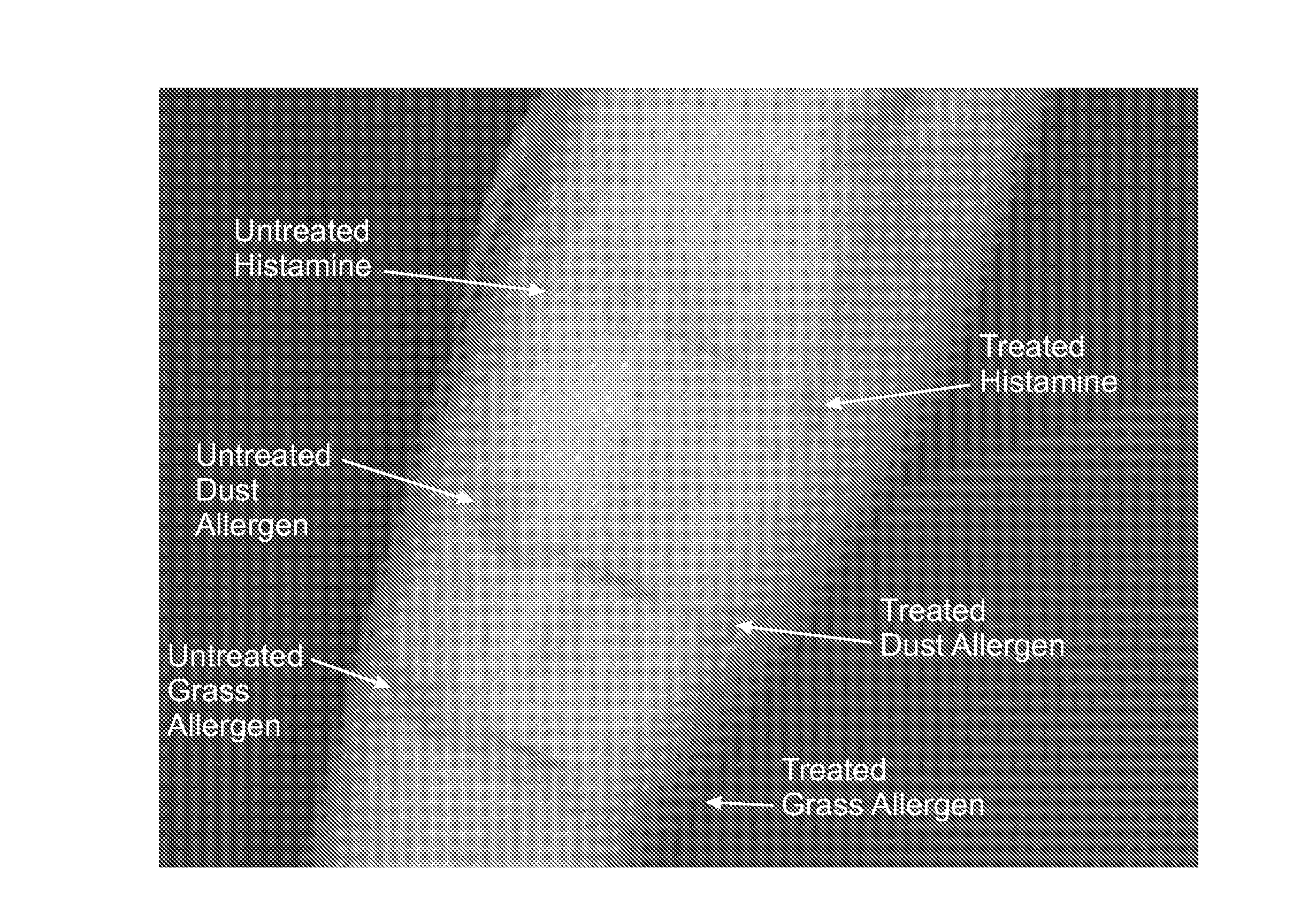
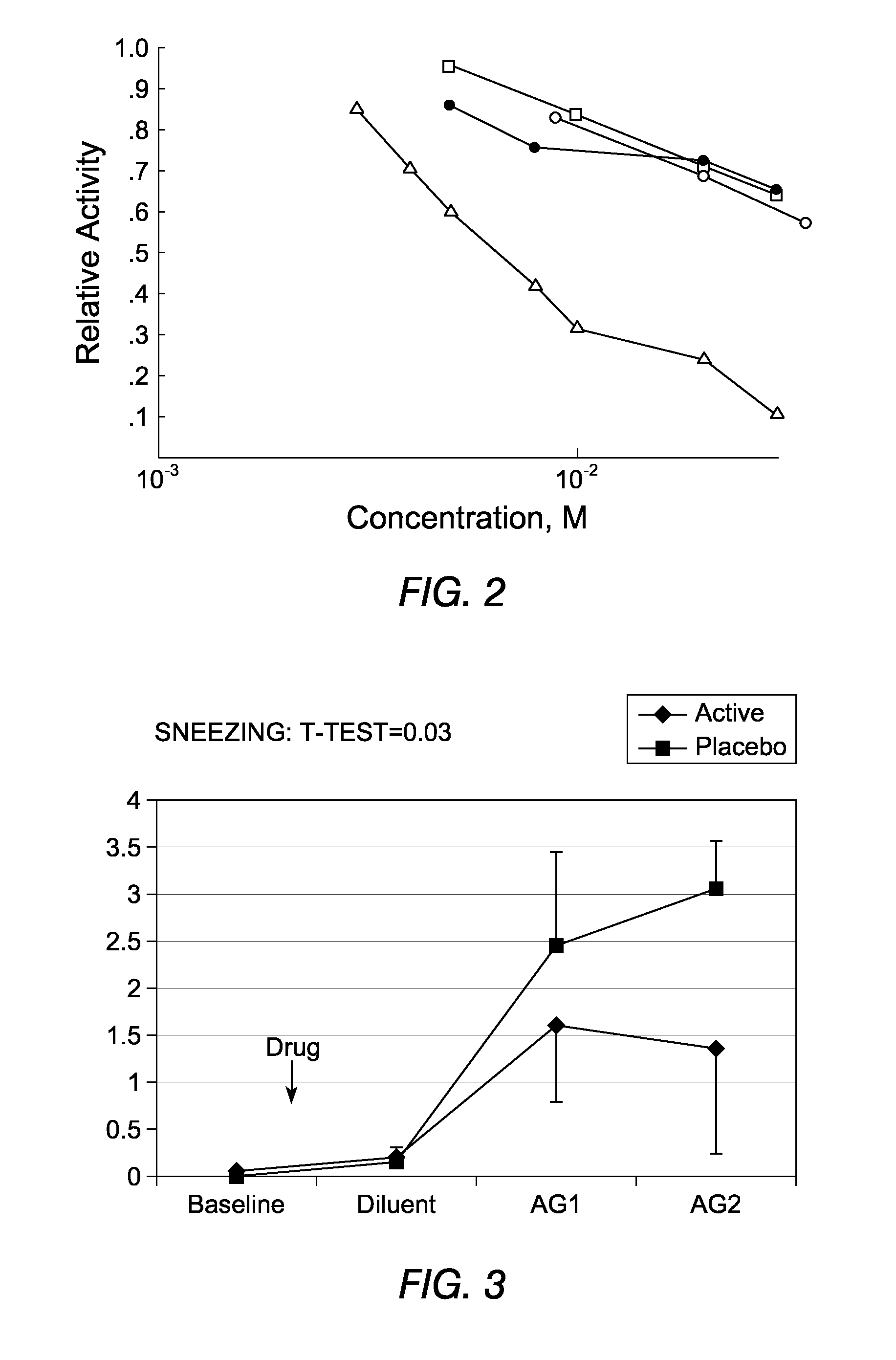
[0140]The patient suffers from allergic rhinitis. He is prescribed a topical composition comprising an X-ray contrast media that is free of EDTA and Ca-Na EDTA. He applies the composition to the nasal mucosa. The composition comprises CM in a concentration of 150 Mg I / ml to 350 Mg of I / mL. Follow up reveals that the administration of the composition improves the allergic condition.
example 2
[0141]The patient suffers from allergic rhinitis. He is prescribed a topical composition comprising an X-ray contrast media that includes EDTA or Ca-Na EDTA and also a sufficient amount of zinc to allow zinc to act as an ACE enzyme. The composition comprises CM in a concentration of 150 Mg I / mL to 350 Mg I / mL. The composition also includes zinc in a concentration of 0.0004 M to 0.001 M. The patient applies the composition to the nasal mucosa. Follow up reveals that the administration of the composition improves the allergic condition.
example 3
[0142]The patient suffers from allergic conjunctivitis. She is prescribed a composition comprising an X-ray contrast media that is free of EDTA and Ca-EDTA and the composition is suitable for use in the eye. She applies the composition topically to the eye as a drop or gel formulation. The composition comprises CM in a concentration of 150 Mg I / mL to 350 Mg I / mL. Follow up reveals that the administration of the composition improves the allergic condition.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com