Probiotic composition for use in the treatment of bowel inflammation
a technology of probiotics and bowels, applied in the field of probiotic compositions for use in the treatment of bowel inflammation, can solve the problems of no medical cure for ibd, delayed development and stunted growth, and reduced life quality, and achieve the effect of mild gut inflammation and mild colitis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
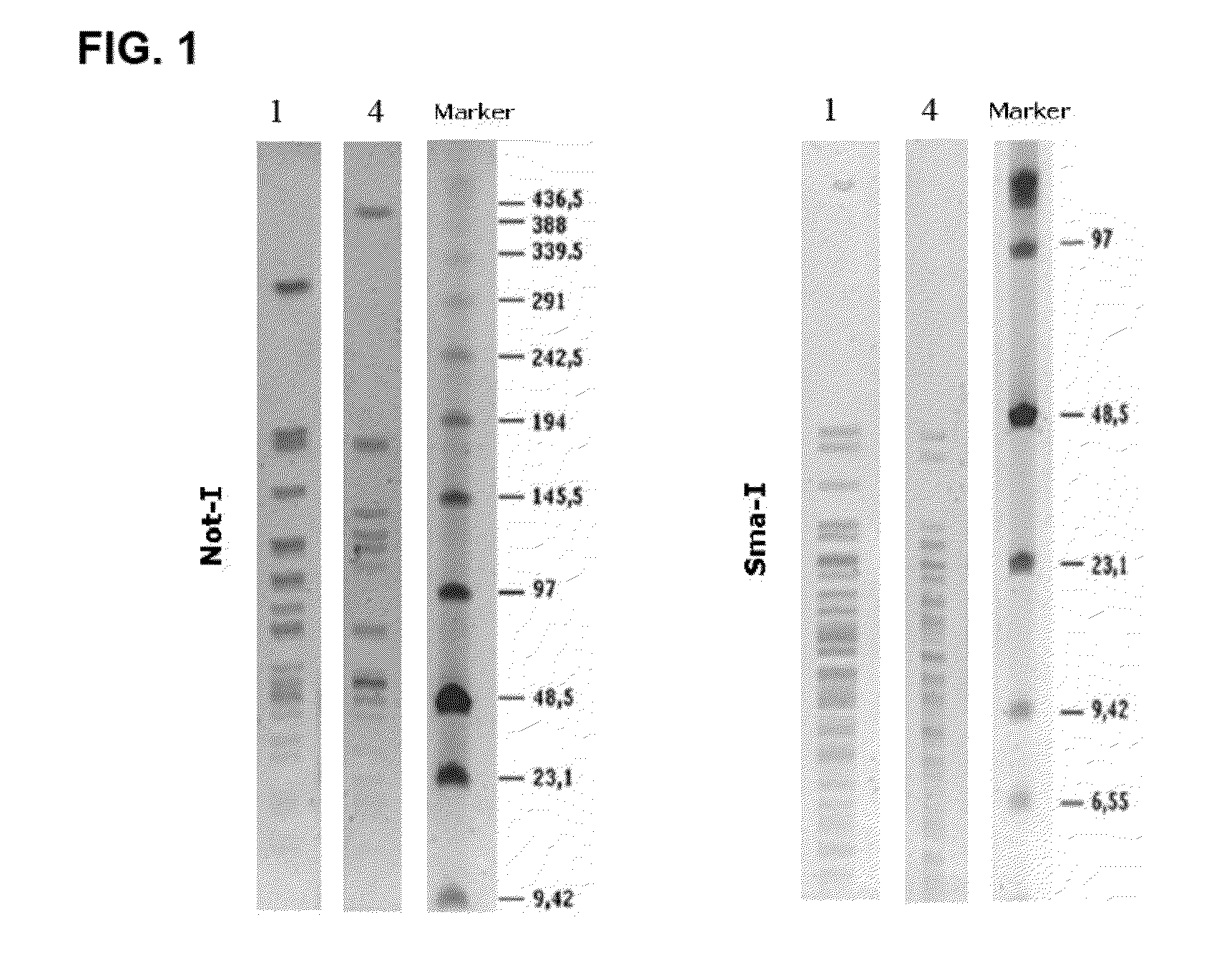
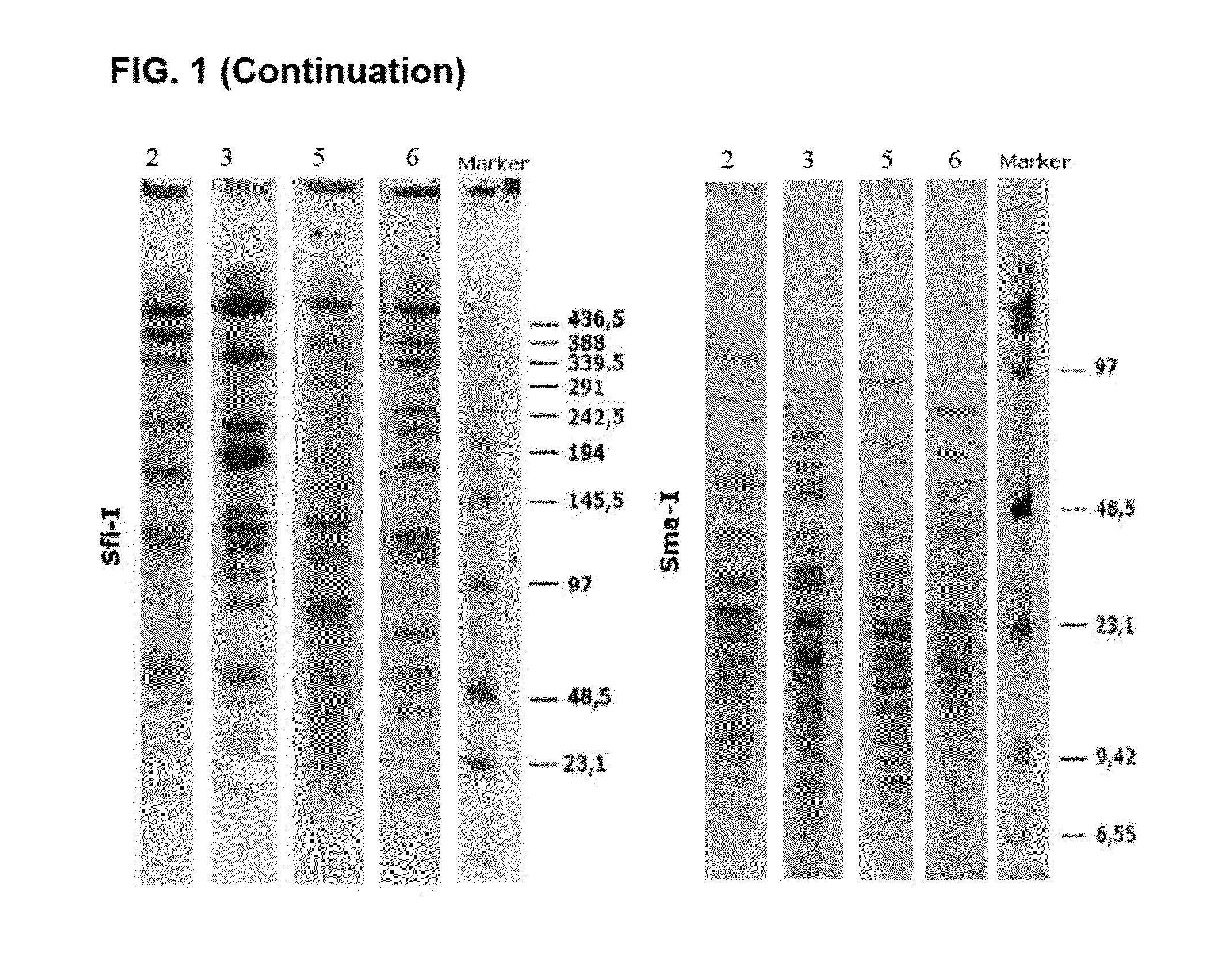
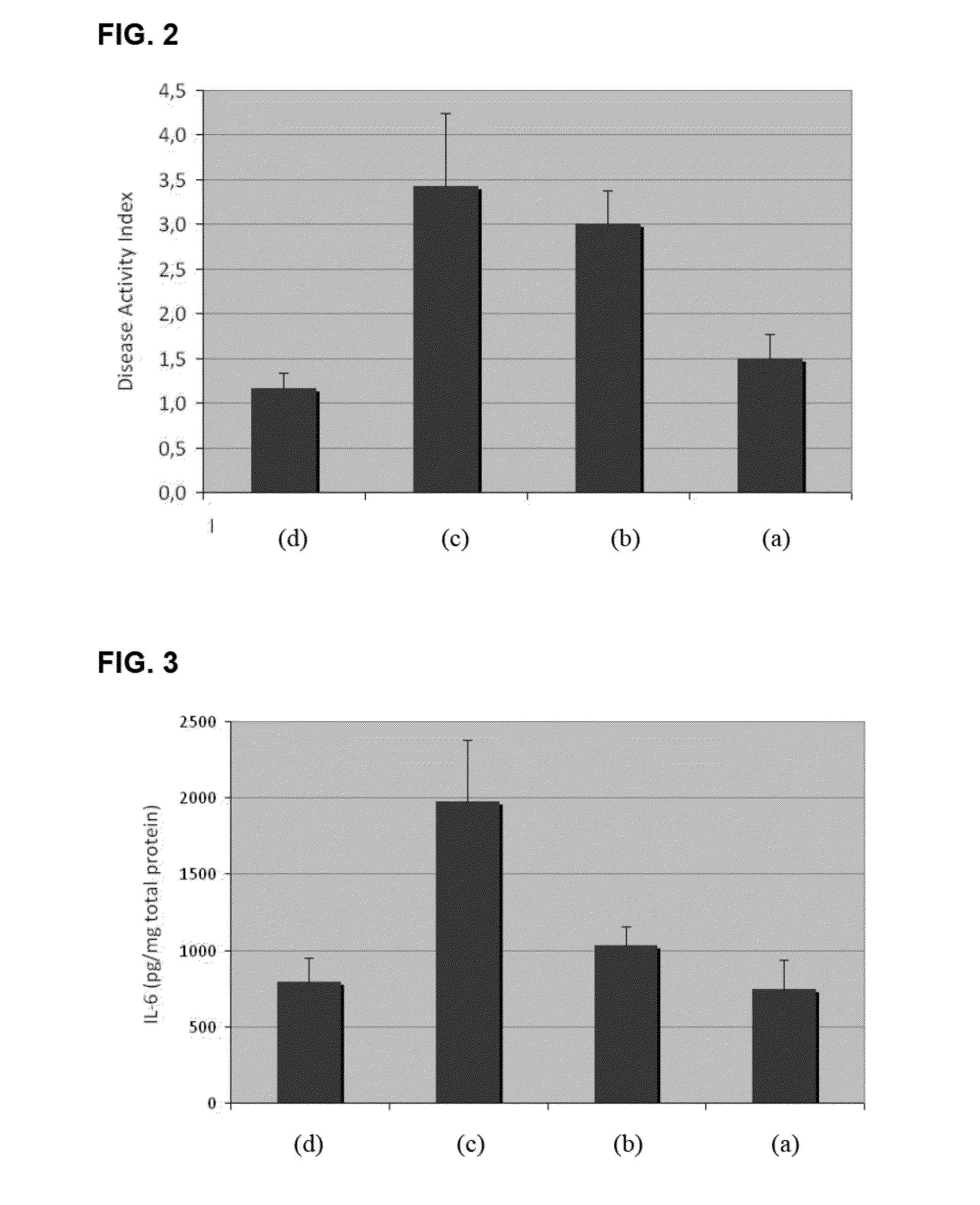
[0062]The following sections describe the characterization of the strains of the invention, their specific probiotic features and their physiological effects on the gastrointestinal and immune systems. As used hereinafter, strain F1033 corresponds to Pediococcus acidilactici CETC 7483, strain F2064 to Lactobacillus plantarum CECT 7484, and strain F2076 to Lactobacillus plantarum CECT 7485.
1. Isolation of Microorganisms
A) Methods
[0063]For isolation of microorganisms, fresh stools and saliva (Daniel C. et al., 2006) were collected from 0-5 year-old children and dissolved in PBS buffer (pH 7.4), aliquoted and plated on MRS supplemented with various antibiotic combinations. Strains were cultured under microaerophilic conditions (5% CO2) at 37° C. Incubation time depended on the growth rate, but run normally from 24 h to 3 days. Gram staining was carried out in order to get a first identification. Once grown, isolated strains were stored by lyophilisation in PBS 0.1× with 15% skim milk p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com