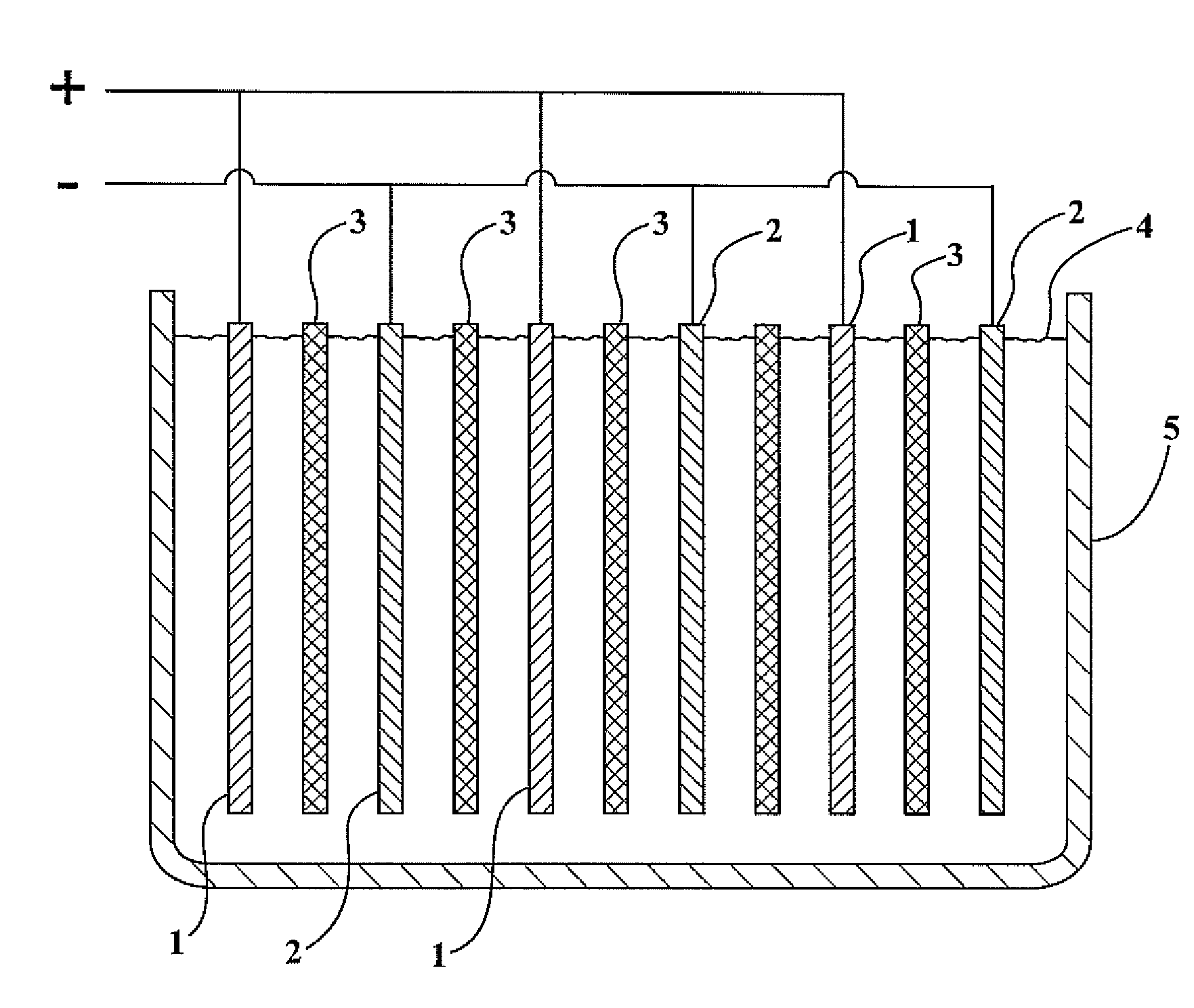
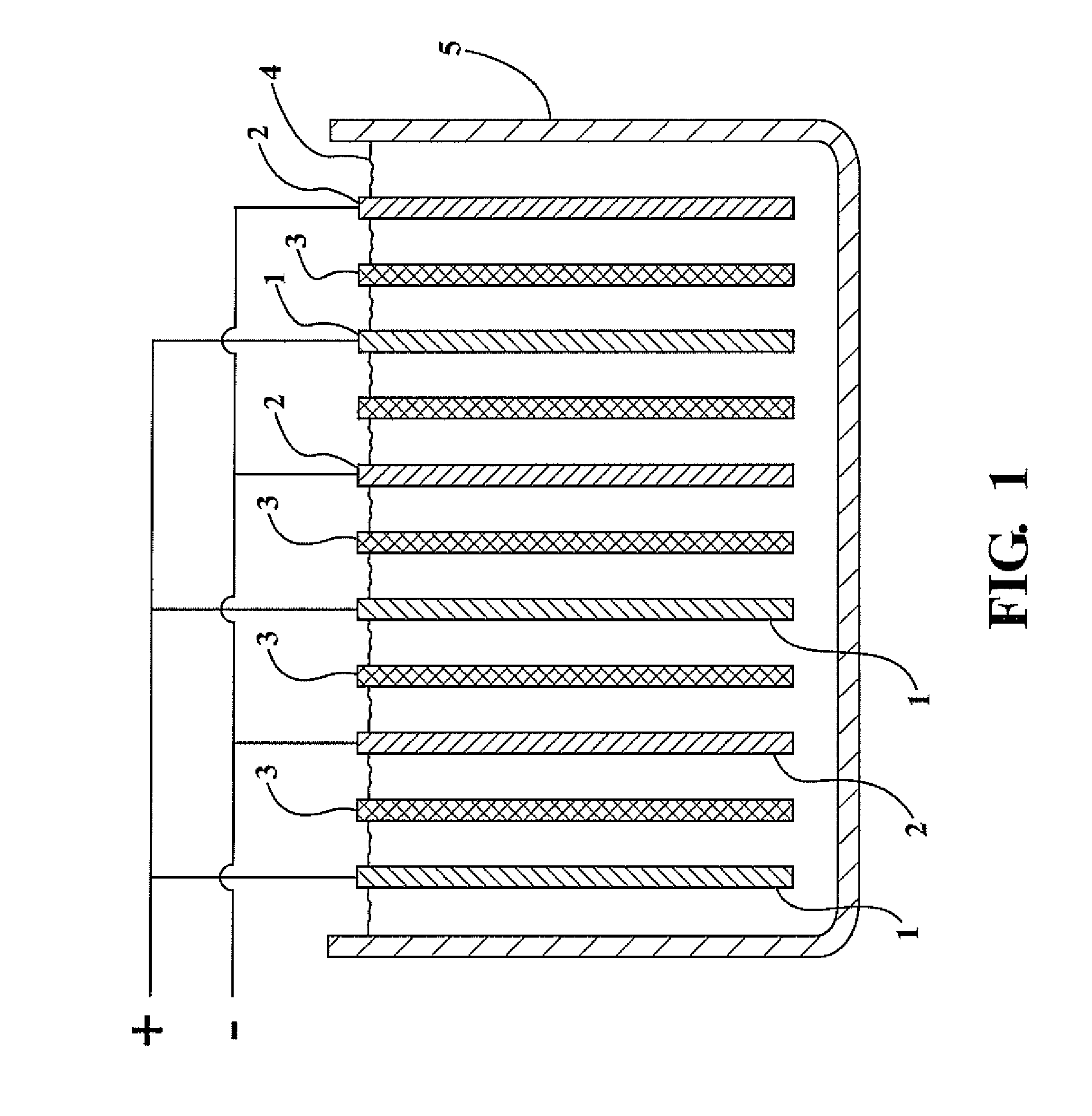
Lead-zinc battery
a zinc battery and lead-zinc technology, applied in the direction of secondary cells servicing/maintenance, cell components, electrochemical generators, etc., can solve the problems of limited energy density, limited cycle life, and extremely corrosive electrolyte, so as to improve electrical conductivity, prevent short circuit, and maximize electrode surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024](1) A glass jar 4.5 in. high was used for the cell. A plastic divider kept the electrodes apart. The positive electrode was a strip of lead 1.5 in. wide by 5 in. high. The negative electrode was a sheet of zinc 1.75 in. wide by 3.5 in. long, which had been recovered from a dry cell. The electrolyte was prepared by dissolving 48.1 gm. of sodium bisulfate monohydrate, reagent grade in 200 ml. water. After charging the cell for 8 minutes at 3.0 volts, an open circuit potential of 2.65 volts was observed. The cell was discharged, producing a current of 100 milliamps through a flashlight bulb. After repeated cycling, the electrodes showed no corrosion.
[0025](2) The same cell as used in example (1) was employed except the negative electrode comprised a rod of zinc 99.9999 percent (metals basic) pure. The electrolyte was formulated by dissolving 33.0 gm. of potassium bisulfate, 97% in 200 ml. of water. After charging the cell for 11 minutes at 3.0 volts, an open circuit potential of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy density | aaaaa | aaaaa |
| energy densities | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com