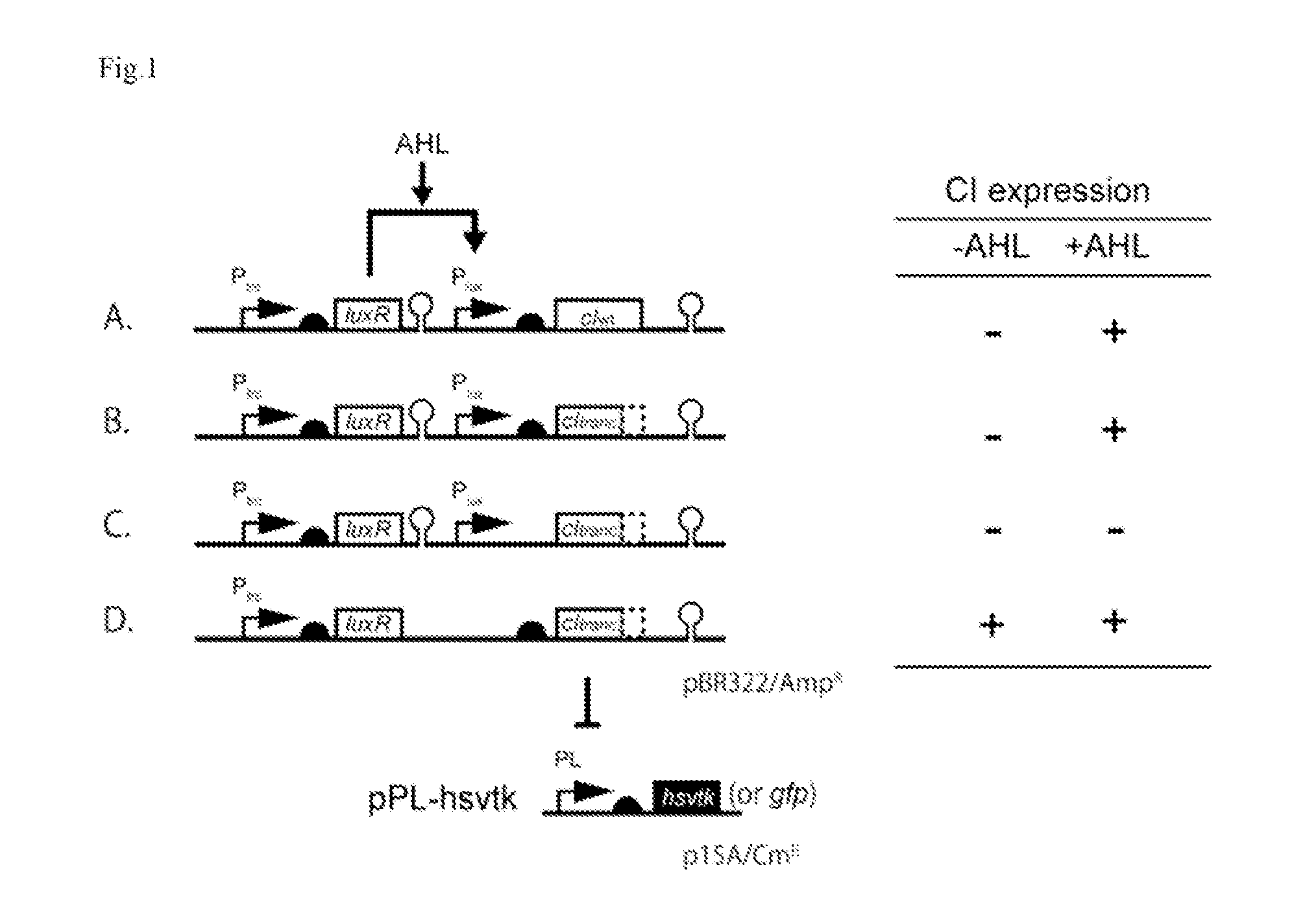
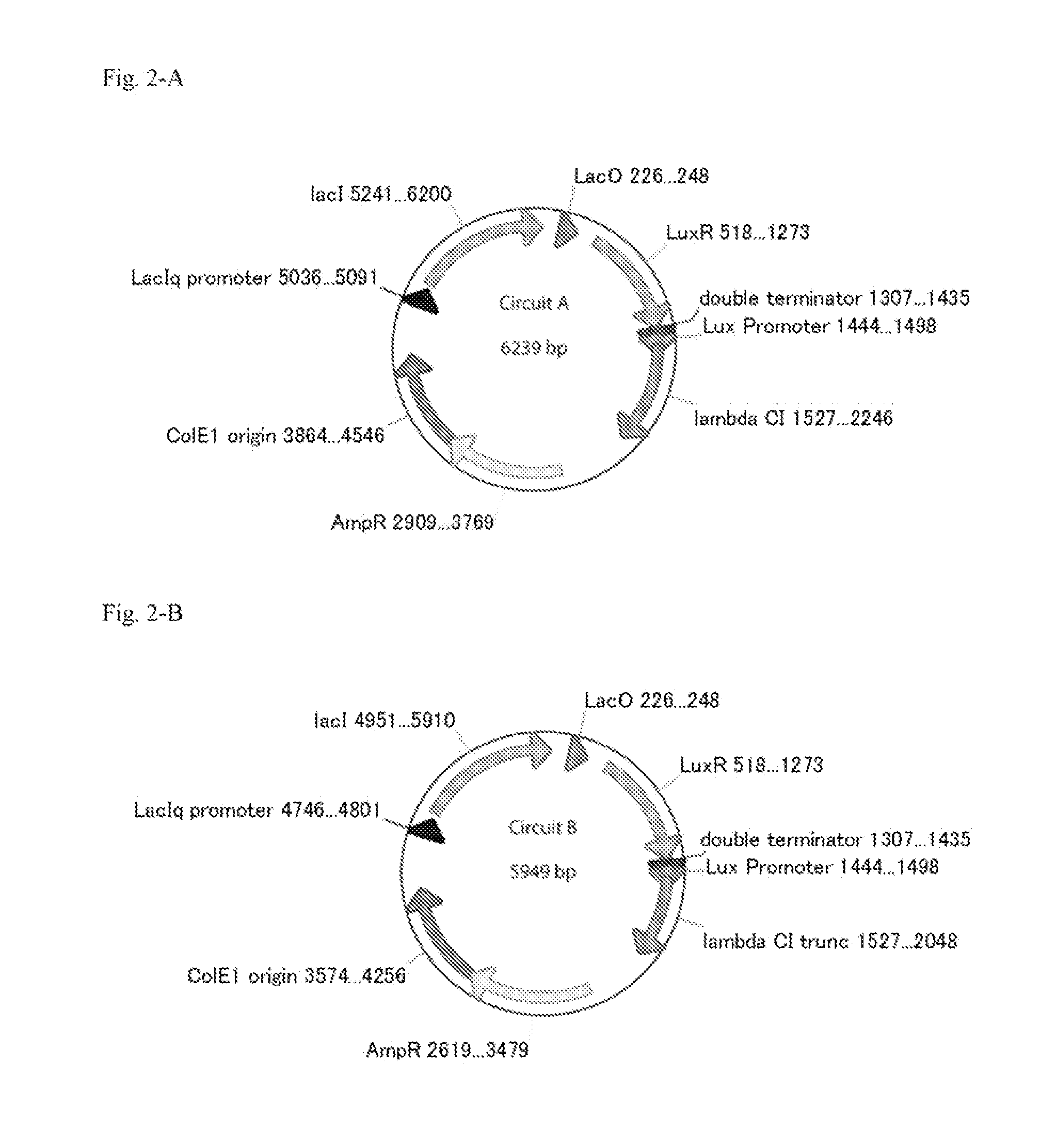
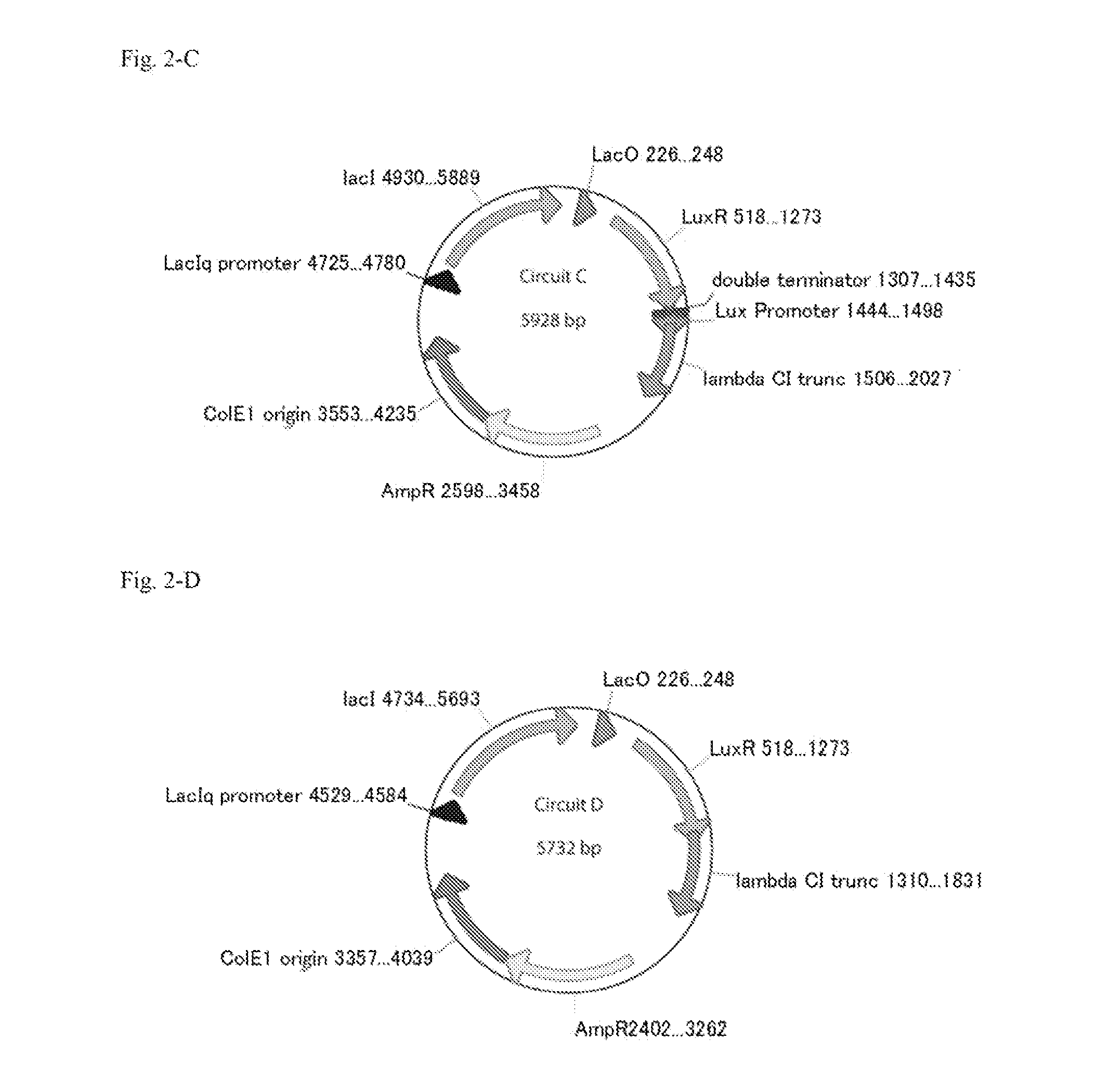
Method For Rapidly Developing Gene Switches And Gene Circuits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental example 1
[0172]An OFF selection method for a genetic switch and a genetic circuit was established.
[0173]First, with the use of mutation frequency in a low-concentration dP medium as an indicator, TK, thymidine monophosphate kinase (Tmk), and nucleotide diphosphate kinase (Ndk) were overexpressed. As a result, it was found that, as with other nucleoside analogs, the rate-limiting step of the incorporation of dP into DNA was the first phosphorylation catalyzed by TK (unpublished data). Next, some tk genes of different origins were tested. As a result, hsvTK showed the highest dP incorporation efficiency.
[0174]With the TK-deficient E. coli strain JW1226, human herpes virus derived thymidine kinase (hsvTK) was expressed by transfecting pTrc-hsvtk (FIG. 3), and a relationship between the concentration of dP and the survival rate of cells (viable cell count) was investigated.
[0175]First, JW1226 cells transfected with pTrc-hsvtk were cultured in 2 mL of LB medium containing 100 μg / mL of ampicillin ...
experimental example 2
[0180]An ON selection method for a genetic switch and a genetic circuit was established.
[0181]With the TK-deficient strain of E. coli JW1226, a human herpes virus derived thymidine kinase (hsvTK) was expressed by transfecting pTrc-hsvtk (FIG. 3), and a relationship between the concentration of 5FdU and the survival rate of cells (viable cell count) was investigated.
[0182]First, JW1226 cells transfected with pTrc-hsvtk were diluted with 1 mL of positive selection medium so as to achieve an OD600 of 0.002 (approximately 106 cells / mL), and cultured at 37° C. for 12 hours while being rotated at 200 rpm. The positive selection medium used was medium containing tryptone at 2% w / v, NaCl at 0.5% w / v, dT at 10 μg / mL, adenosine at 1 μg / mL, and 5FdU at 0 to 25 μg / mL. After the culture, a portion of the resultant culture was collected and inoculated in an LB plate containing ampicillin, and the number of colonies grown was counted as a viable cell count.
[0183]In the TK-deficient E. coli strain ...
experimental example 3
[0187]The rapidity of the OFF selection method established in Experimental Example 1 was studied.
[0188]First, TK-deficient cells transfected with pTrc-hsvtk (FIG. 3) so as to constantly express hsvTK were cultured under shaking at 37° C. and 200 rpm until an OD of 0.05 (approximately 3×107 cells / mL) was achieved. The resultant was dispensed at 1 mL each, dP was added (final concentration: 1 μM), and culture was continued at 37° C. After a lapse of 5, 30, or 60 minutes, 20 μL of the culture (approximately 106 cells) were collected and inoculated in an LB plate containing ampicillin, and the number of colonies grown was counted as a viable cell count.
[0189]In the sample to which a dP shock had been applied, the viable cell count was almost zero at any action time (FIG. 6). On the other hand, when the dP shock was not applied, the viable cell count increased. From the results, it was found that the OFF selection was sufficiently completed by an operation within up to 5 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com