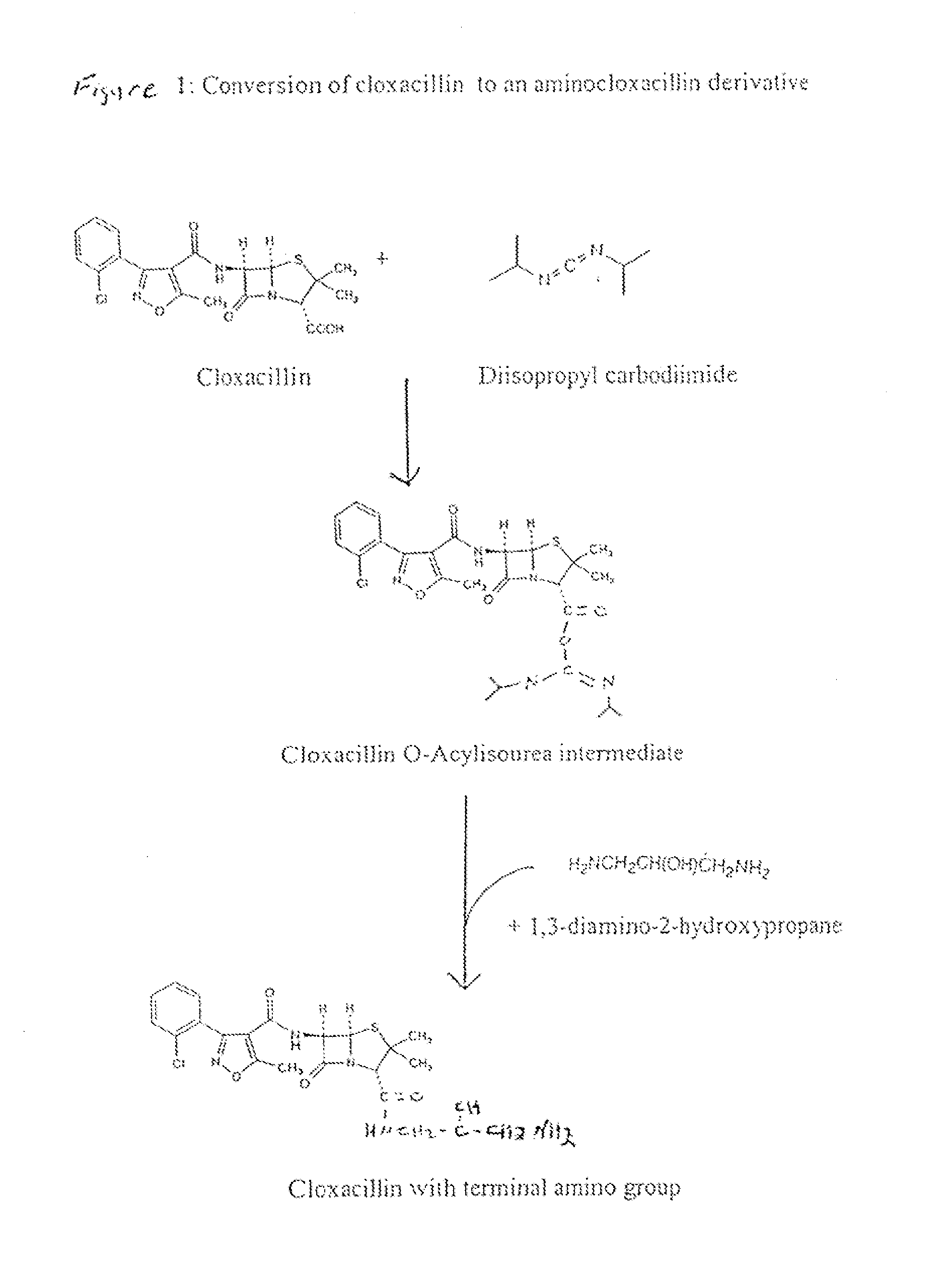
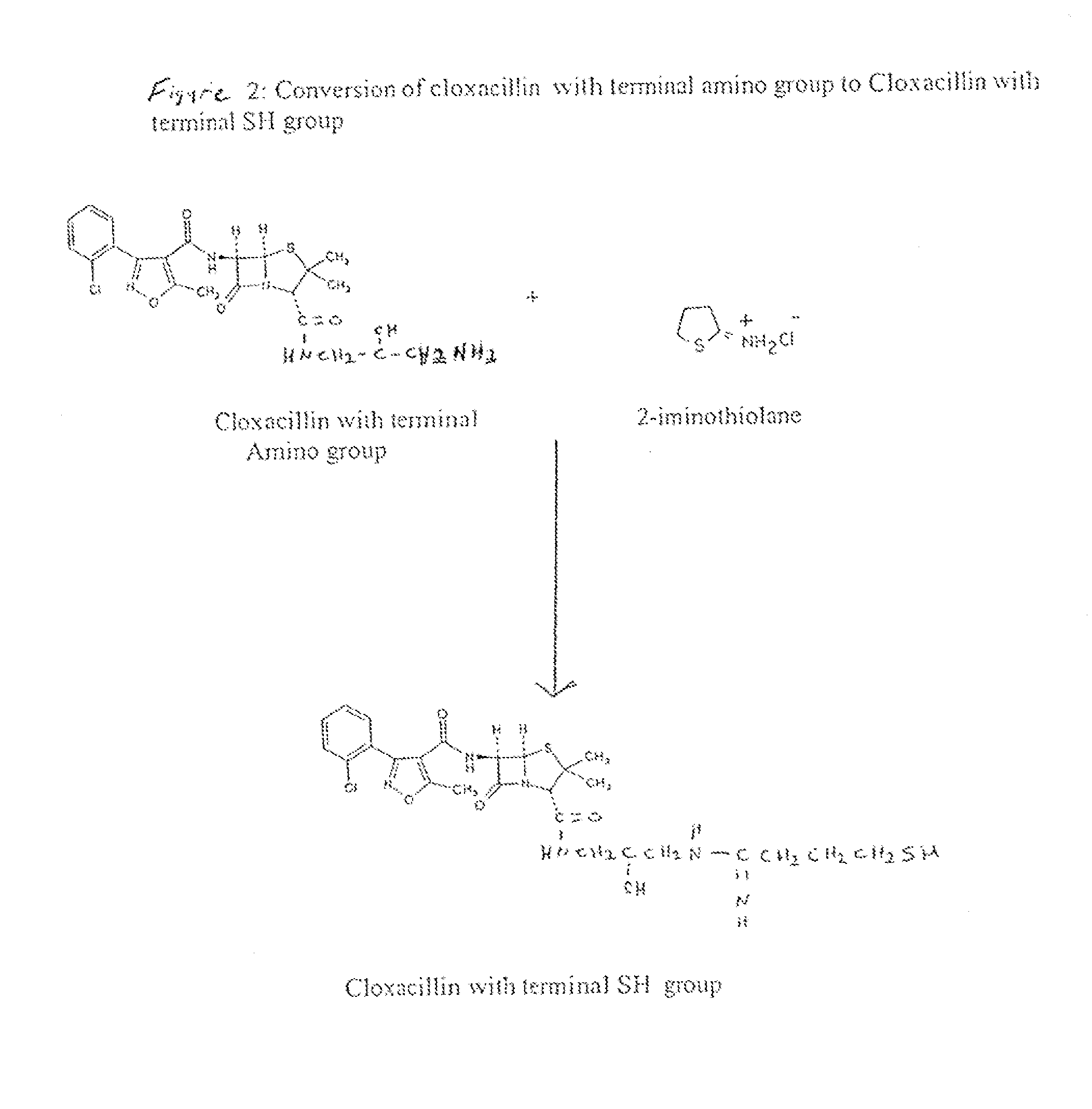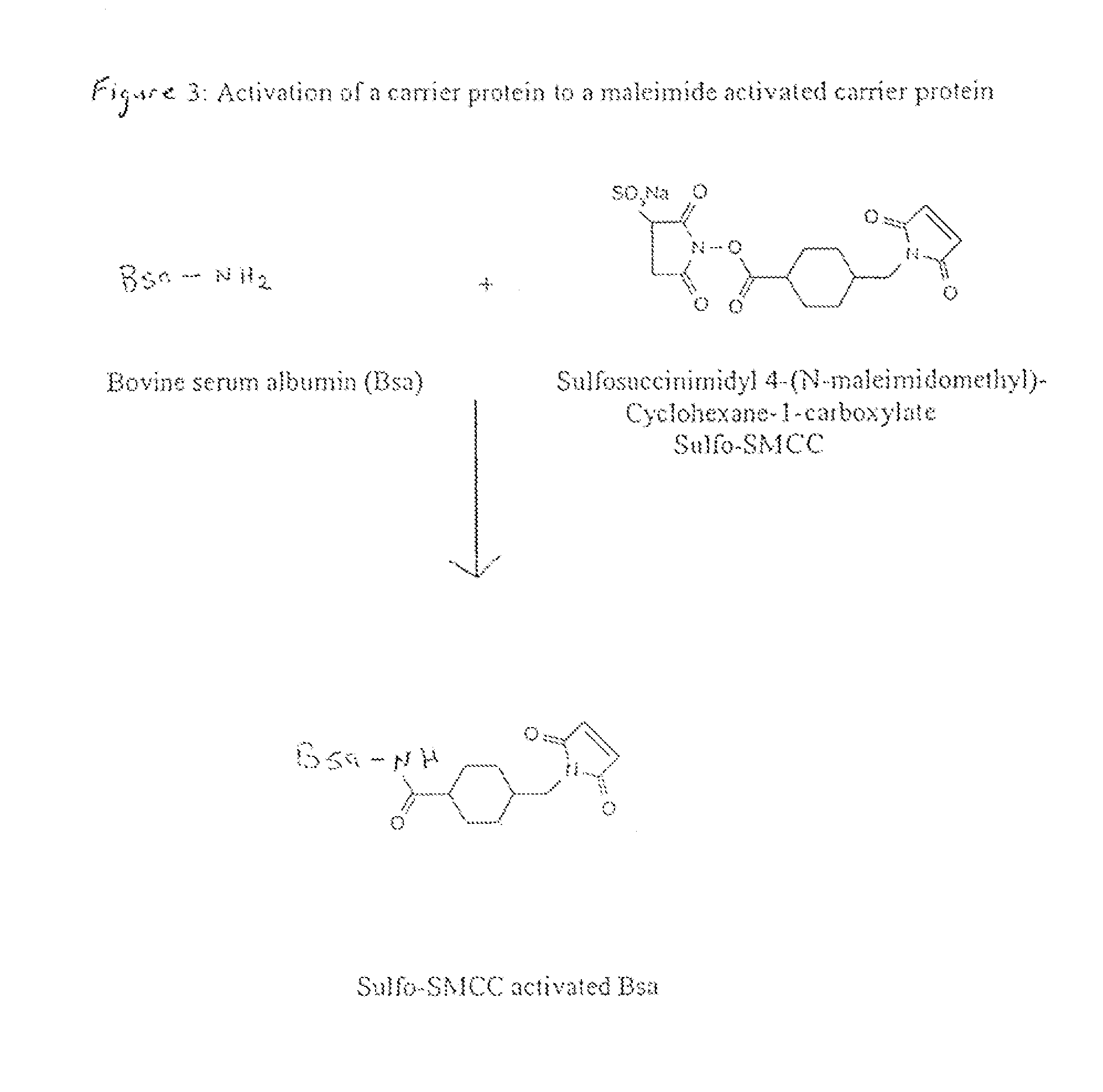Method of Attaching a Ligand to a Test Strip
a technology of ligands and test strips, applied in the field of attaching small molecule ligands to, can solve the problems of clogging pores, inhibiting flow, limited amount of used, etc., and achieve the effects of increasing the test sensitivity to the analyte, and reducing the amount of related analyte availabl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0053]The invention will now be described by the following example: Lateral-flow test for six beta-lactams in milk at safe level.
[0054]The three-line test for detection of penicillin-G, amoxicillin, ampicillin, cloxacillin, ceftiofur and cephaparin at safe level consists of two test zones and one control zone.
[0055]The first test zone solution, consisting of a ceforanide-BSA conjugate at 2-4 mg / ml in 10 mM sodium phosphate, pH 6.9, containing 8% sucrose, 2-6 mg / ml BSA, 0.01% BIO-TERGE® (BIO-TERGE is a registered trademark of StepHan Chemical company of Northfield, Ill.) and PROCLIN® 5000 (155 μl / L) (PROCLINE is a registered trademark of Rohm and Haas Company of Philadelphia, Pa.) was sprayed onto nitrocellulose using a BIODOT® sprayer. The mixture was sprayed 1.6 cm above the bottom edge of the nitrocellulose at a rate of 0.8 μl / cm.
[0056]To make the second test zone solution, 1 g of cloxacillin was dissolved in approximately 20 ml anhydrous DMSO. Further, 500 mg of N-hydroxysulfosuc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com