Sustained drug delivery implant
a technology of brimonidine tartrate and implanted ocular cavity, which is applied in the direction of prosthesis, eye treatment, drug composition, etc., can solve the problems of ocular morbidity, retinal detachment, and certain limitations of intraocular use of brimonidine tartra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0060]Example intraocular implants containing brimonidine tartrate or brimonidine free base and a biodegradable polymer matrix were created and tested for their release and degradation properties. The brimonidine tartrate or brimonidine free base was first weighed and blended with PLA and / or PLGA polymers in a Turbula mixer for 30 minutes. The resulting powder blend was then fed to the Haake extruder by a force feeder. The extruded filaments were cut to implants with a target weight, e.g., 857 μg or 800 μg to deliver 300 μg brimonidine tartrate or 400 μg brimonidine free base per implant. Implants were loaded into 25G applicators and gamma-sterilized at 25 to 40 kGy dose. The potency per implant was confirmed by a HPLC assay.
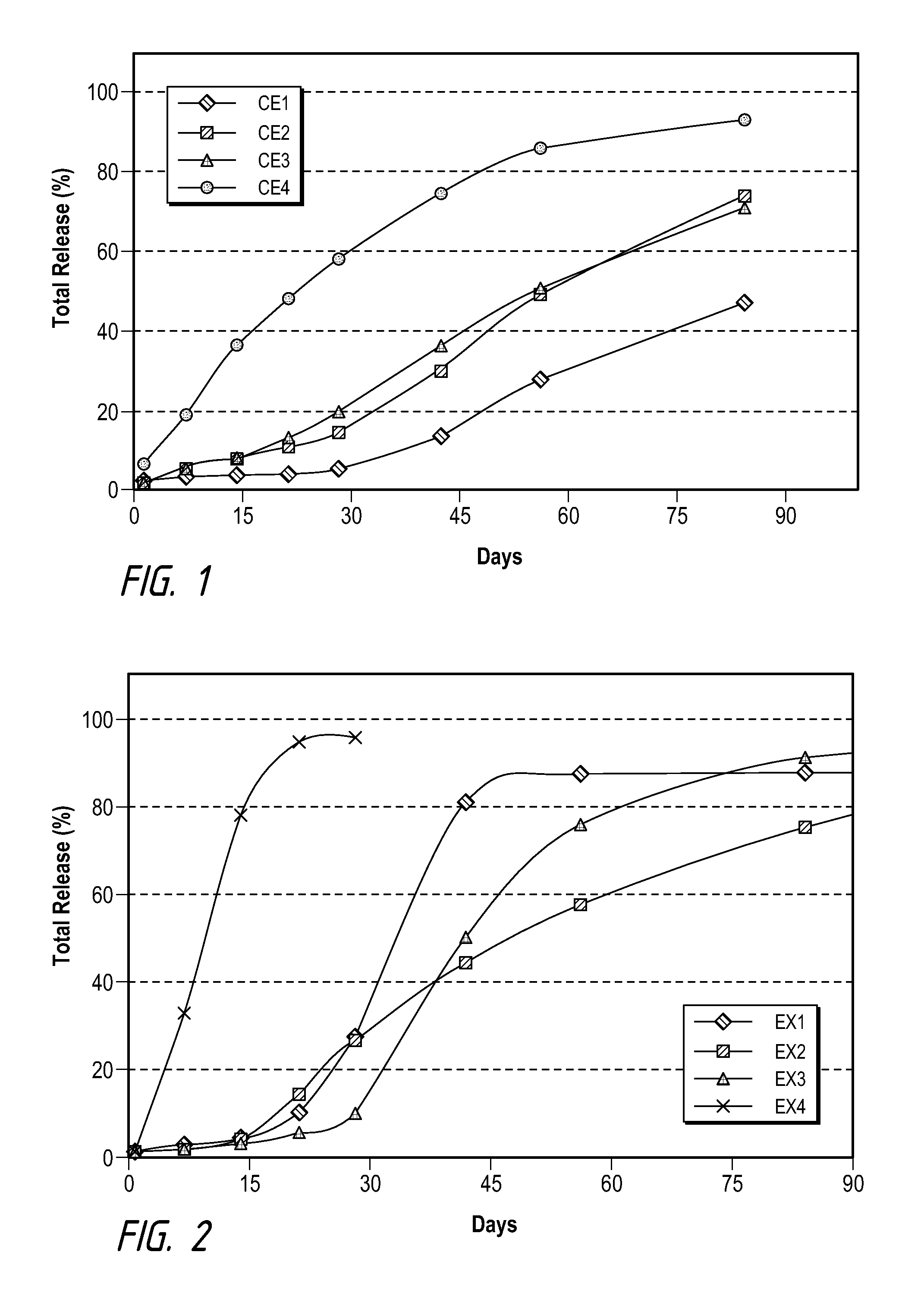
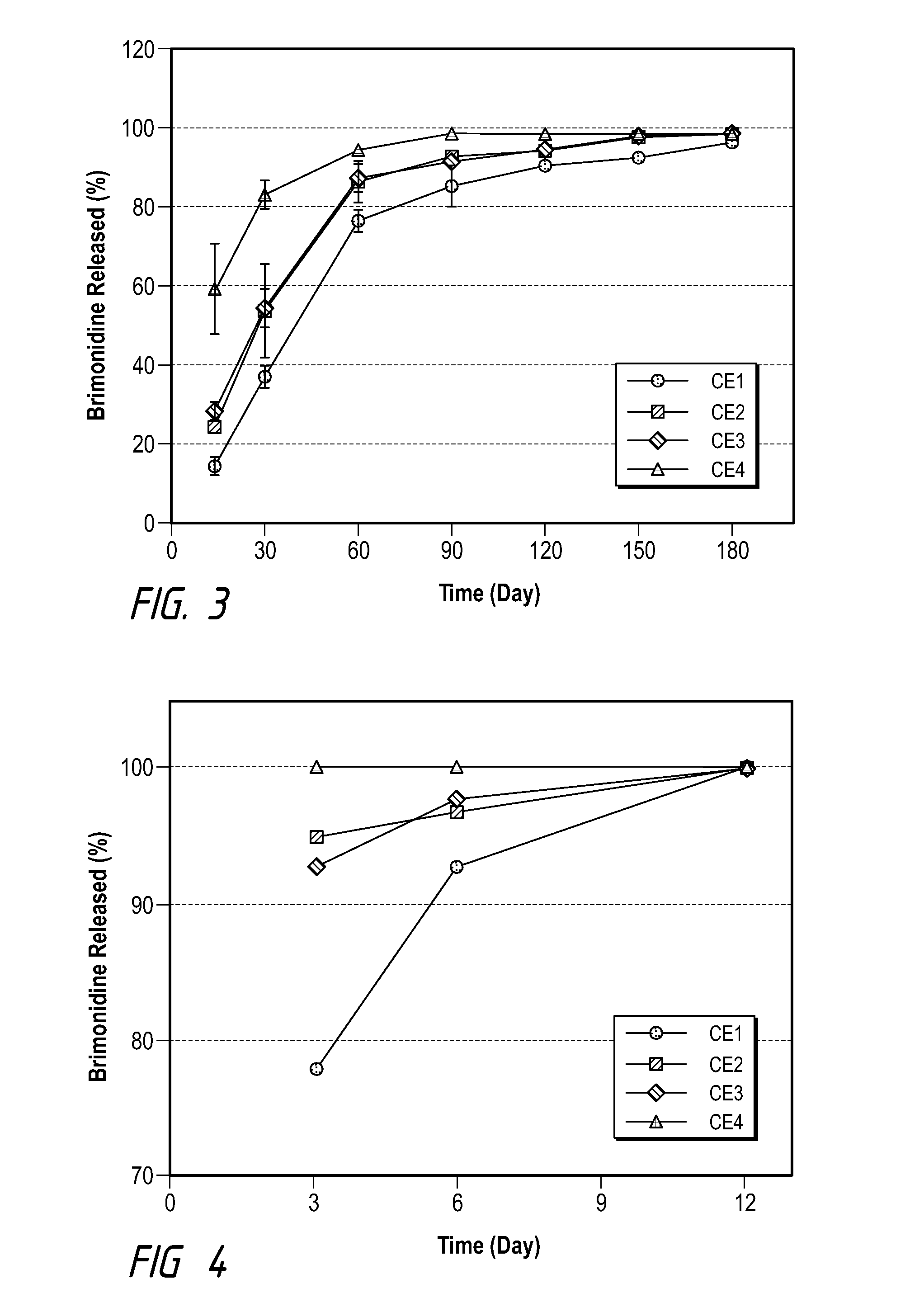
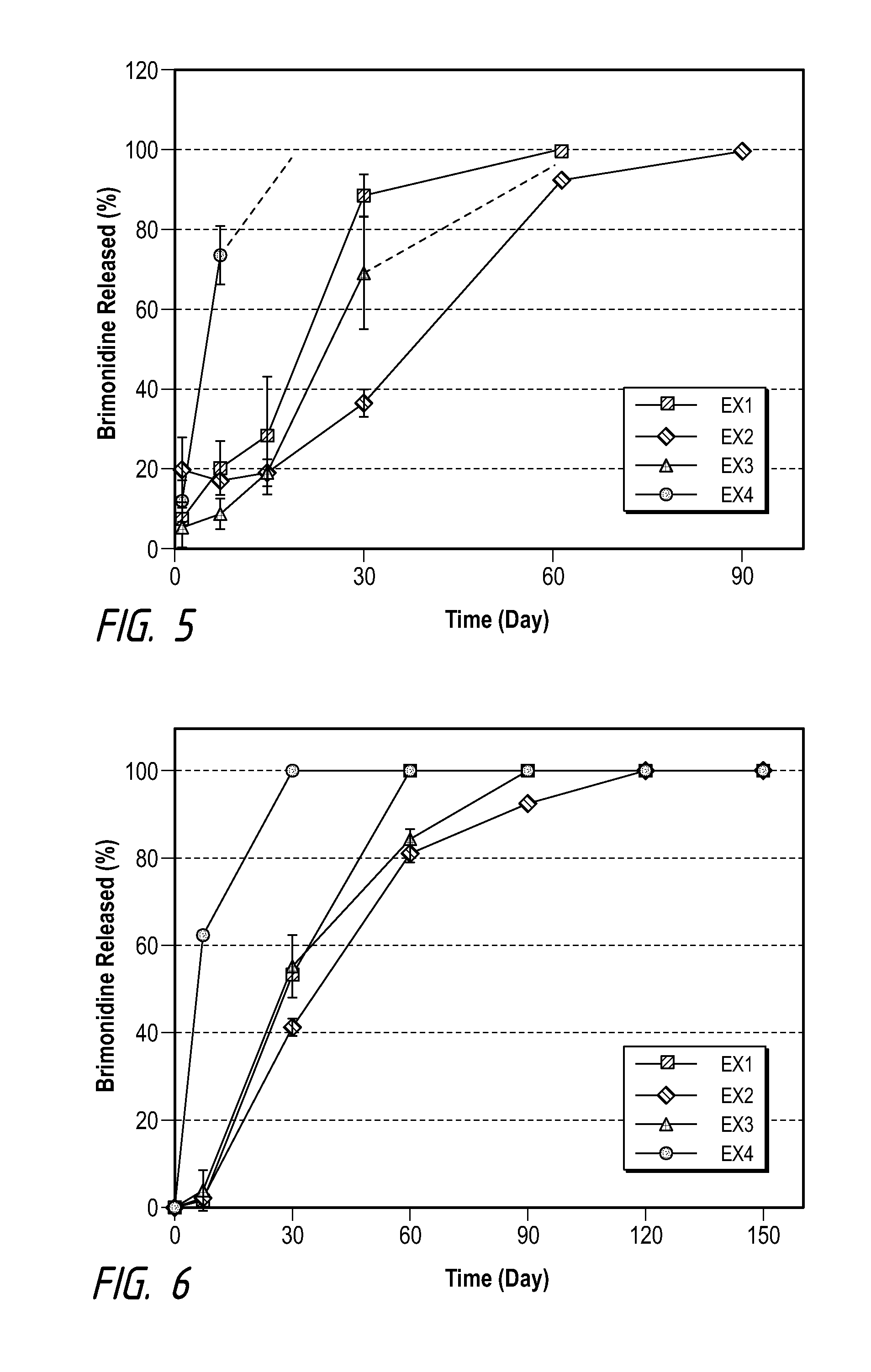
[0061]Examples and Comparative Examples of formulation compositions using brimonidine tartrate (as Comparative Examples 1-4) and brimonidine free base (Examples 1-4) as the drug are shown in Tables B and C, and their drug release profiles are shown in FIGS. 1 an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com