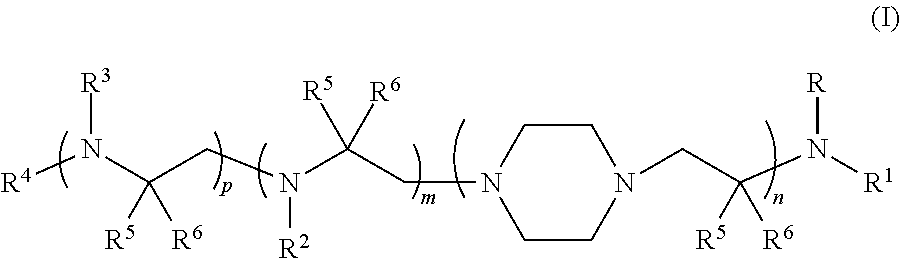
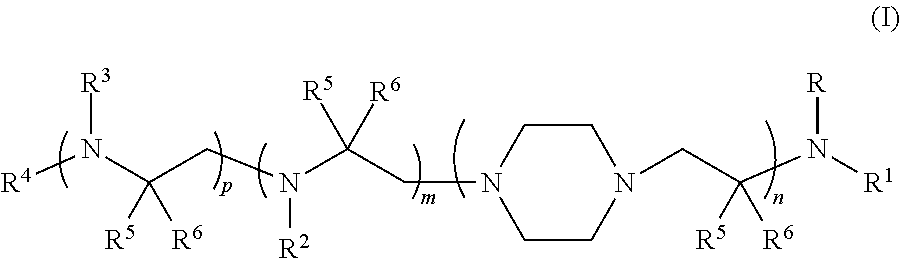
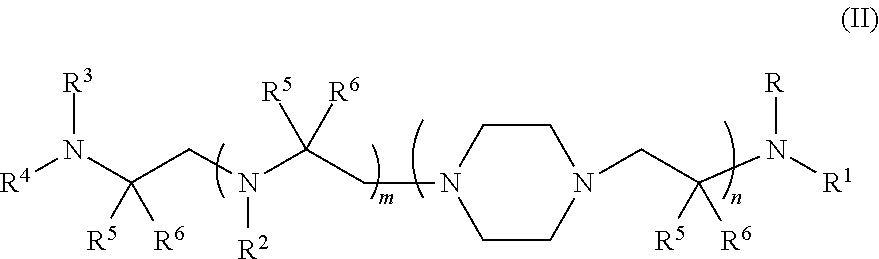
Aminoalcohol compounds and their use as zero or low VOC additives for paints and coatings
a technology of amines and additives, applied in the field of amines, can solve the problems of poor scrub resistance, high odor of such amines, and unsuitability for low odor paint, and achieve the effect of reducing the content of volatile organic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
3,3′-((((2,3-dihydroxypropyl)azanediyl)bis(ethane-2,1-diyl))bis(azanediyl))bis(propane-1,2-diol) (DETA-Glyc)
[0076]A 3-neck round bottom flask equipped with a magnetic stirrer, nitrogen blanket, thermocouple controlled heating mantle and addition funnel is charged with diethylene triamine (103 g, 1 mole, 1.0 equivalent) and 100 mL of water. A slight exotherm is observed during the mixing of the amine and water. Once the exotherm subsides, the addition funnel is charged with 96% pure Glycidol (192.5 g (174 mL), 2.5 moles, 2.5 equivalent) and added very slowly to the amine / water solution. The reaction is extremely exothermic and therefore, slow addition of the alkylene oxide will control the reaction temperature. The reaction has been run at 25° C. (control temperature by slow addition and ice-bath), 60° C. (control temperature by slow addition and ice-bath) and at 85° C. (control temperature by slow addition). In all cases, the reaction products are similar. Addition of Glycidol to th...
example 2
1,1′-((((2-hydroxypropyl)azanediyl)bis(ethane-2,1-diyl))bis((2-aminoethyl)azanediyl))bis(propan-2-ol) (TEPA-3PO)
[0077]Using tetraethylene pentamine (0.5 mol) and propylene oxide (1.5 mol) as starting materials and following analogous procedures to those described above, the title compound may be prepared. LC / MS analyses show component masses ranging from 363 to 537 daltons. IR shows OH and NH2 at 3294 cm−1, aliphatic CH at 2962 and 2815 cm−1, and aliphatic CH2 and CH3 at 1456, 1370, 1334, 1293, 1133, 1061, 1013, 944, and 842 cm−1. 1H-NMR shows a broad singlet at 3.836 ppm (2.5 Hs), a complex multiplet at 2.724-2.308 ppm (22 Hs), and a complex multiplet at 1.168-1.092 ppm (9 Hs). 13H-NMR showed 13 signals at 77.175-73.531 ppm, 4 signals at 68.508-67.271 ppm, 7 signals at 65.667-62.911 ppm, 10 signals at 60.326-56.966 ppm, single signals at 51.935 and 50.255 ppm, and 6 signals at 31.960-30.842 ppm.
example 3
7,13-bis(2-hydroxypropyl)-4,7,10,13,16-pentaazanonadecane-2,18-diol (TEPA-4PO)
[0078]Using tetraethylene pentamine (0.5 mol) and propylene oxide (2.0 mol) as starting materials and following analogous procedures to those described above, the title compound may be prepared. The IR and NMR analysis results are similar to those obtained for TEPA-3PO.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com