Method for the treatment of fatty liver disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Example 1
Isolation of Exemplary Cyclohexenone Compounds
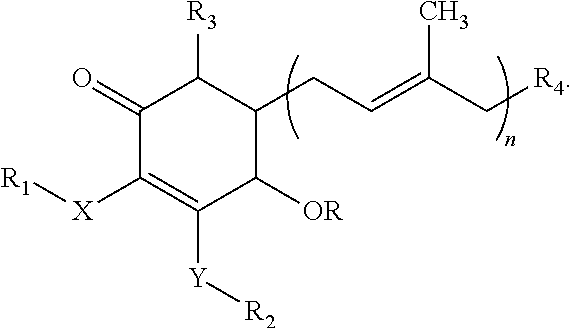
[0056]100 g of mycelia, fruiting bodies or mixture of both from A. camphorata were placed into a flask. A proper amount of water and alcohol (70-100% alcohol solution) was added into the flask and were stirred at 20-25 degrees Celsius (° C.) for at least 1 hour. The solution was filtered through a filter and a 0.45 μm (micrometer) membrane and the filtrate was collected as the extract. The filtrate of A. camphorata was subjected to a High Performance Liquid chromatography (HPLC). The HPLC was performed using a RP18 column, methanol (A) and 0.1˜0.5% acetic acid (B) as the mobile phase, with gradient of 0˜10 min in 95%˜20% B, 10-20 min in 20%˜10% B, 20˜35 min in 10%˜10% B, 35˜40 min in 10%˜95% B, at a flow rate of 1 ml / min. The effluent was monitored with a UV-visible detector.
[0057]The fractions at 25˜30 min were collected and concentrated to yield Compound 1, 4-hydroxy-2,3-dimethoxy-6-methyl-5 (3,7,11-trimethyl-dodeca-2,6,10-tri...
example 2
Reduction of Fatty Liver Condition by Compound 1 (4-hydroxy-2,3-dimethoxy-6-methyl-5 (3,7,11-trimethyl-dodeca-2,6-10-trienyl)-cyclohex-2-enone)
[0063]In order to simulate an unhealthy diet tendency in humans, such as excess consumption of high caloric food, the example constructs an animal model using rats fed a high fat diet to evaluate the effects of chronic liver injury. Thereafter, the effects can be revealed through biochemical assays to prove the fatty liver reduction of exemplary compounds provided herein such as Compound 1,4-hydroxy-2,3-dimethoxy-6-methyl-5(3,7,11-trimethyl-dodeca-2,6-10-trienyl)-cyclohex-2-enone (the test compound).
[0064]The assay simulates liver disease caused by a high fat diet through a “Metabolic Syndrome” model. That is, the model is different from conventional chemical induced models caused by toxic components such as CCl4. This model is distinguishable from the models caused by virus or alcohol.
[0065]The establishment of a long term high-fat diet is d...
example 3
Reduction of Fatty Liver Condition by the Composition Comprising Compound 1 and Ergosterol
[0089]Except for the testing groups and treatment schedule, the materials and processes of Metabolic Syndrome” model, Histology analysis, Whole blood and plasma biochemistry and Fatty Liver Disease Caused by Non-Chemical Injury are similar to those stated in Example 2.
[0090]In control group (group A), six rats were fed with vehicle (corn oil from Sigma chemical co.) by means of stomach tubes. Six rats in group B were orally administered vehicle and the test compound (Compound 1) at a dose of 48 mg / kg twice a day (96 mg / kg per day) and ergosterol at a dose of 12 mg / kg twice a day (24 mg / kg per day). Six mice in group C were orally administered vehicle and the test compound (Compound 1) at a dose of 48 mg / kg twice a day (96 mg / kg per day).
[0091]Table 1 below shows the study timetable summary.
TABLE 1The timetable of the studyTimeProcessDay 0BirthDay 2STZ treatmentDay 28Feeding high fat dietDay 62R...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com