Treatment of metabolic disorders in equine animals
a metabolic disorder and equine technology, applied in the field of veterinary medicine, can solve the problems that insulin-related disorders have a severe and life-threatening impact on the health of equine animals, and no satisfactory treatment is currently available for metabolic disorders, so as to achieve optimal dosing and compliance, attenuate, delay or prevent the progression of metabolic disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Pharmacokinetics (PK) / Pharmacodynamics (PD) of Compound A Single Oral Dosing in Horses
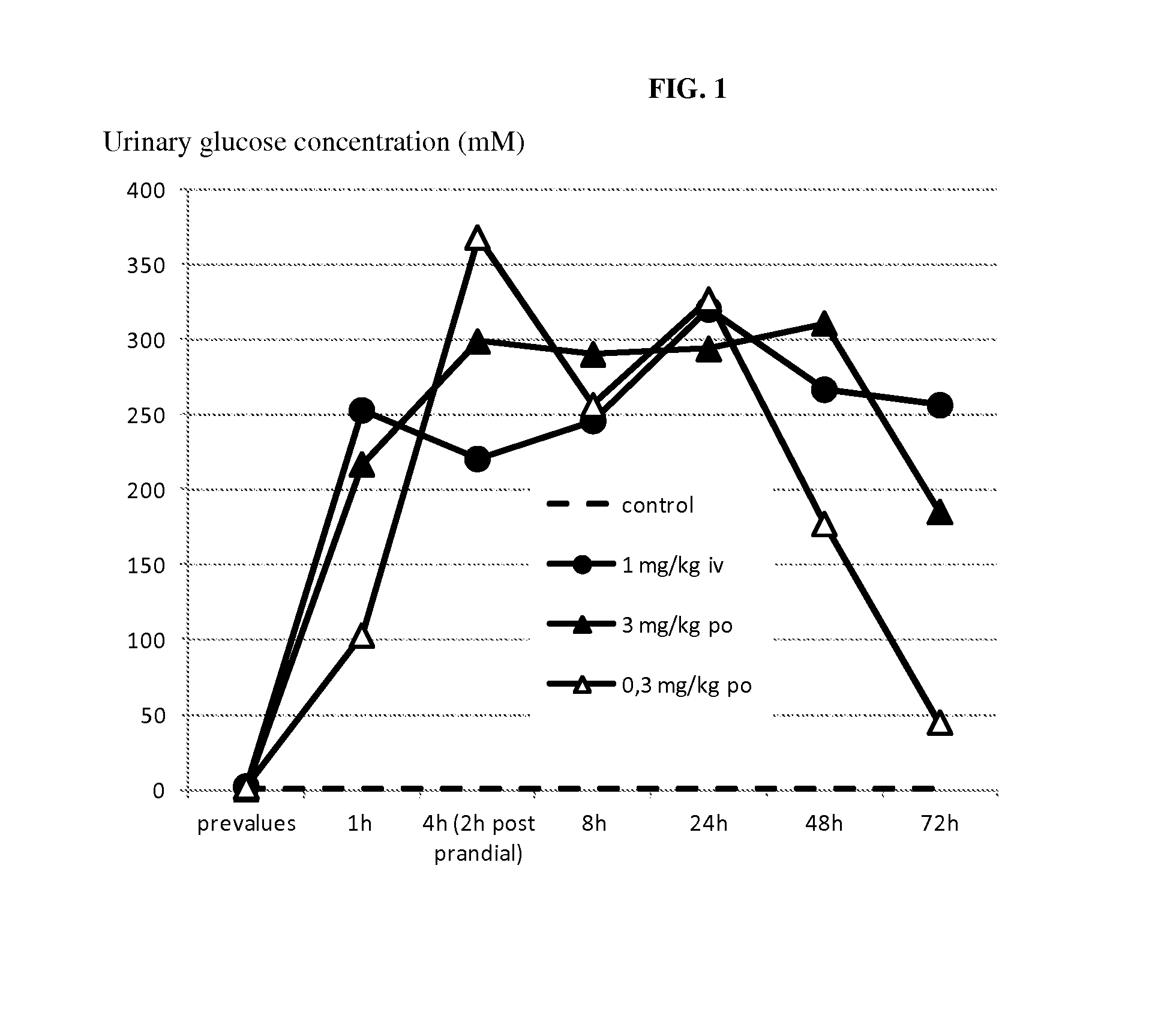
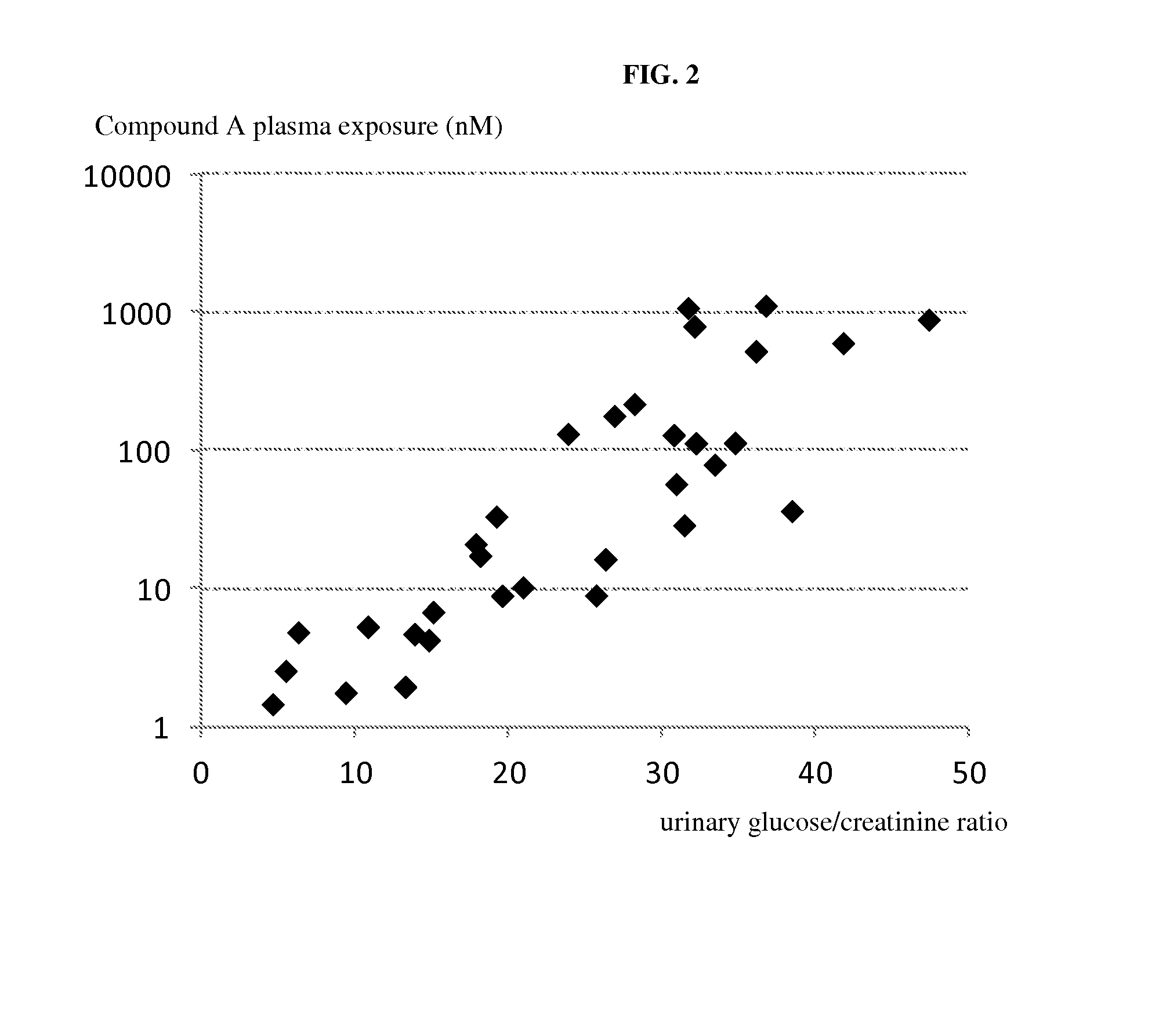
[0212]Compound A was administered to overnight fasted horses. The groups (n=3 per group) received a single oral or intravenous (i.v.) administration of either vehicle alone (purified water, macrogol 15, hydroxystearate) or vehicle containing the SGLT2 inhibitor at a dose of 0.3 mg / kg bodyweight and 3 mg / kg bodyweight orally and 1 mg / kg bodyweight i.v. PK / PD measurements were taken until day 3 after a single administration of compound A or its vehicle.
TABLE 2Pharmacokinetic data, single doseParameter1 mg / kg i.v.0.3 mg / kg p.o.3.0 mg / kg p.o.tmax [hour]mean21Cmax [nmol / L]mean3533867AUC0→∞, mean41251286929752[nmol · h / l]T1 / 2 [hour]mean7.98.58.2
[0213]Pharmacodynamic Data:[0214]A prominent increase of urinary glucose concentration was evident at all doses already 1 h after administration (mean group values: controls 0.6 mmol / L; 1 mg / kg iv-253 mmol / L; 0.3 mg / kgpo-103 mmol / L; 3 mg / kg po-217 mmol / L) and was ...
example 2
The Effect of Compound A on Urinary and Blood Glucose as Well as Glucose Tolerance after Repeated Dosing in Horses
[0218]Compound A was administered to freely fed normoglycemic, hyperinsulinemic, insulin resistant, obese horses, which exhibit an impaired glucose tolerance. The groups (n=4 per group) received a once daily oral administration of either vehicle alone (purified water, macrogol 15, hydroxystearate—0.2 mL / 100 kg and approximately 35 mL of apple sauce) or vehicle containing the SGLT2 inhibitor in increasing doses up to 1 mg / kg for 4 weeks. The treated horses received a daily dose of compound A at 0.1 mg / kg bodyweight for the first 7 days, followed by 0.2 mg / kg bodyweight, from day 20 the dose was increased to 1 mg / kg bodyweight. Urinary glucose and blood glucose were measured. Additionally, to evaluate the glucose tolerance, blood glucose was measured during an oral sugar test (OST, corn syrup 0.15 mL / kg) was performed. Blood was collected via jugular vein catheters. Blood ...
example 3
The Effect of Compound A on Postprandial Blood Glucose in Horses
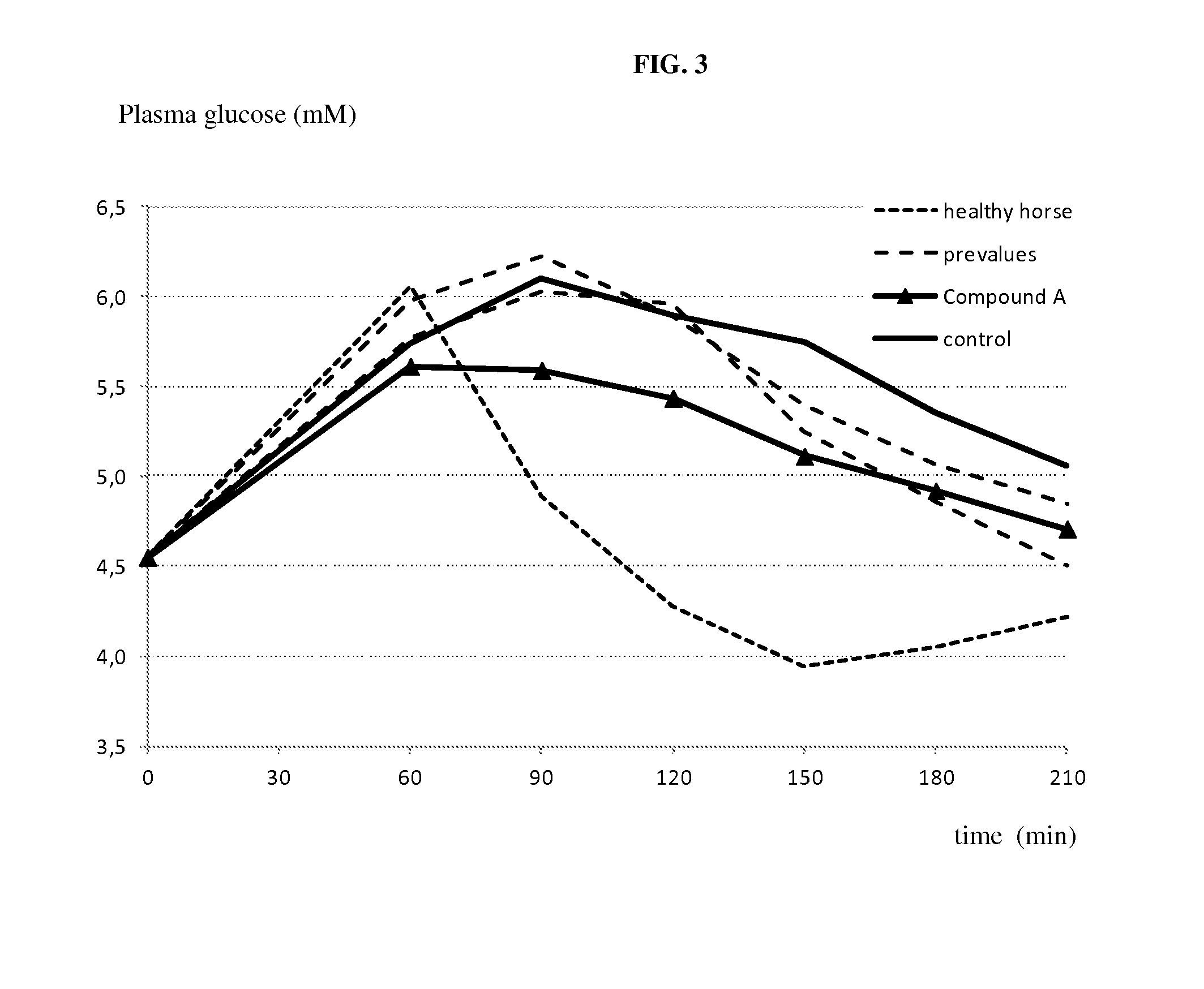
[0224]The following example shows the effect of compound A on postprandial blood glucose in horses. Compound A was administered to overnight fasted horses. The groups (n=3 per group) received a single oral or i.v. administration of either vehicle alone (purified water, macrogol 15, hydroxystearate) or vehicle containing the SGLT2 inhibitor at a dose of 0.3 mg / kg and 3 mg / kg orally and 1 mg / kg i.v. Two hours after compound administration horses were fed a test meal. The postprandial glycaemia is quantified 2 hours thereafter and significantly blunted by all doses of compound A, as shown in 0. Compound A is thus clearly capable of effectively reducing postprandial glucose levels in horses.
[0225]The efficacy of SGLT2 inhibition in accordance with the invention in the treatment of pathological fasting glucose and / or insulin and / or impaired glucose tolerance can be tested using clinical studies. In studies over a shorter or ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com