Conversion of somatic cells into functional spinal motor neurons, and methods and uses thereof
a somatic cell and motor neuron technology, applied in the field of somatic cell transdifferentiation, can solve the problems of not being able to not being able to routinely isolate analogous populations of human neurons or fully study differentiated central neurons, and not being able to refine subtype specific properties and other issues, to achieve the effect of increasing protein expression of brn2, increasing protein expression of myt1l, and increasing protein expression of isl1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0324]In order to determine whether transcription factors can bestow a precise neural subtype identity, we sought factors that could reprogram fibroblasts into spinal motor neurons. Here, the inventors demonstrate induction of motor neurons based on their significant translational utility and because the developmental origins and functional properties of this neural subtype are among the most well understood.
[0325]The inventors demonstrate that when mouse fibroblasts express factors previously found to induce reprogramming toward a generic neuronal phenotype (Vierbuchen et al., 2010), they also respond to components of the transcription factor network that act in the embryo to confer a motor neuron identity on committed neural progenitors. Thus, the inventors demonstrated that forced expression of these transcription factors converted mouse fibroblasts into induced motor neurons (iMNs).
[0326]Importantly, the inventors demonstrated that the resulting iMNs had a gene expression progra...
example 2
11 Factors Convert Fibroblasts into Hb9::GFP+ Cells with Neuronal Morphologies
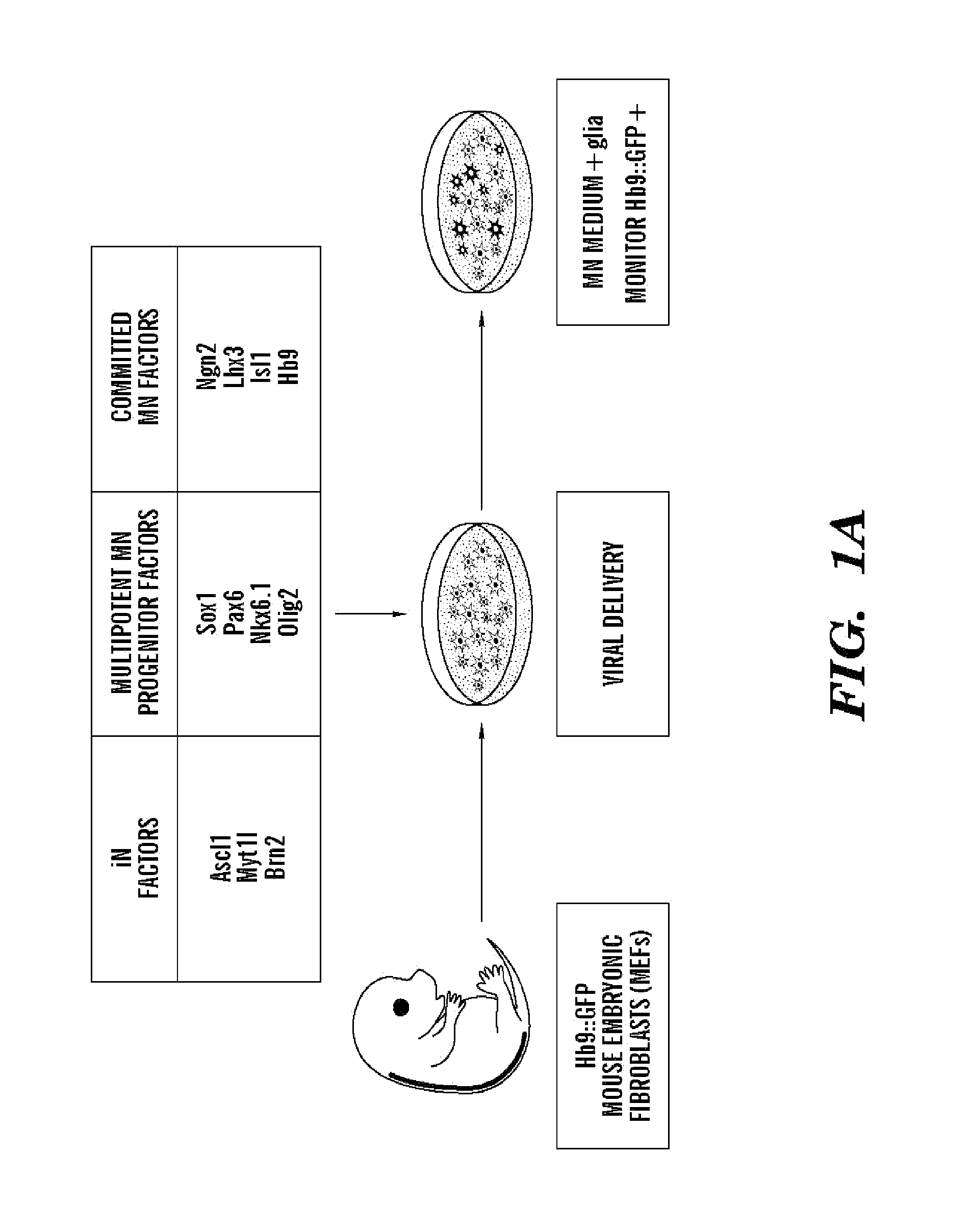
[0327]The inventors assessed if transcription factors known to instruct motor neuron formation during development might also facilitate the conversion of other cell types into motor neurons. The inventors used the literature to select eight candidate transcription factors that participate in varied stages of motor neuron specification (Jessell, 2000). In order to potentially aid the transition toward a neuronal phenotype, the inventors supplemented the motor neuron specification factors with three factors that convert fibroblasts into induced neurons (iNs) of a generic character (Ascl1, Brn2 and Myt1l) (Vierbuchen et al., 2010) (FIG. 1A).
[0328]For reprogramming studies, mouse embryonic fibroblasts (MEFs) were harvested from Hb9::GFP mouse embryos at day E12.5, allowing spinal motor neuron conversion to be monitored. Prior to use, cultures of MEFs were carefully screened for the absence of any contaminating...
example 3
iMNs are Efficiently Induced by 7 Factors
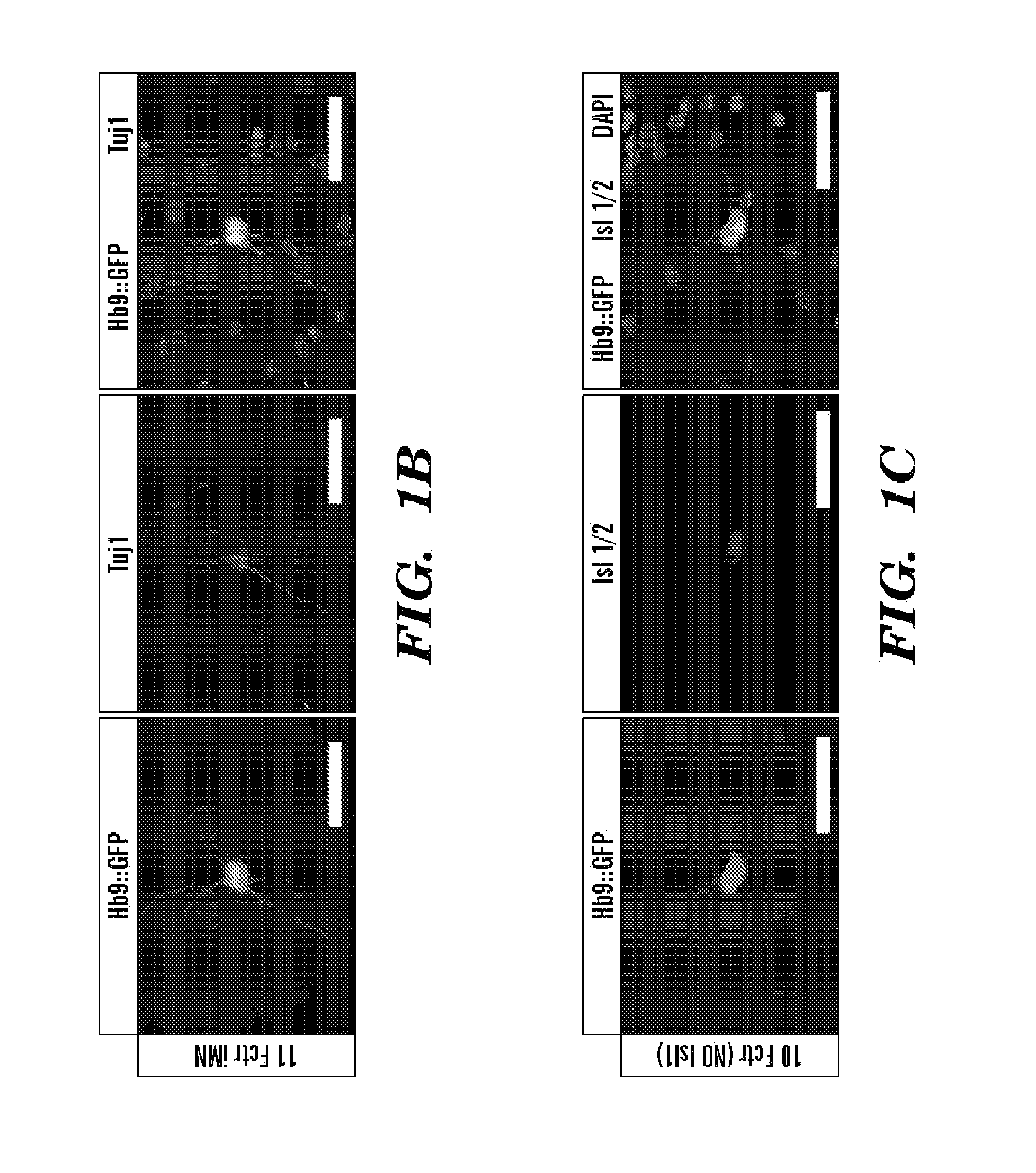
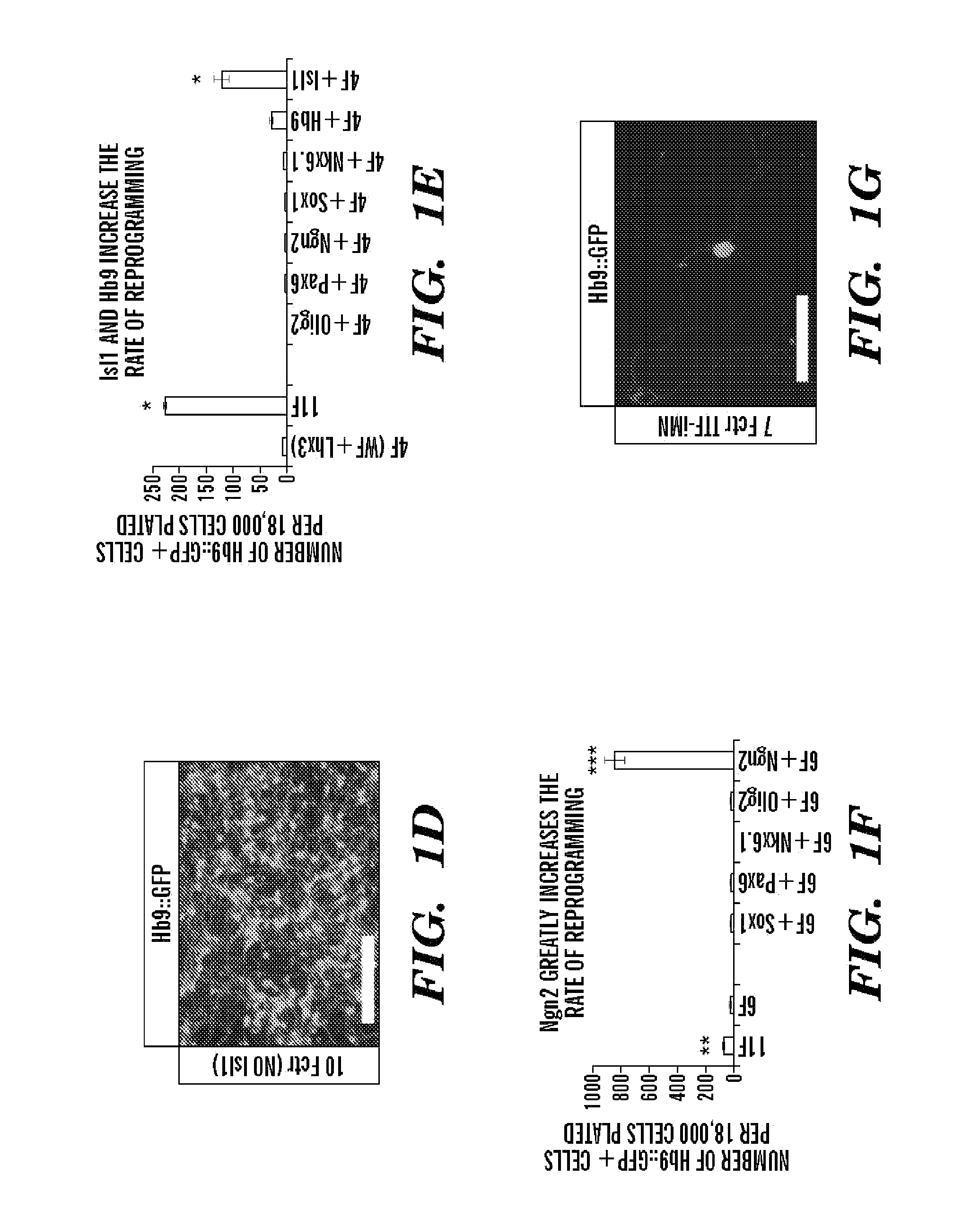
[0330]To determine which of the 11 factors were necessary for generating iMNs, each gene was omitted one at a time (FIG. 8B). Excluding either Lhx3 or Ascl1 eliminated iMN formation. However, reprogramming efficiency was either only slightly reduced or unchanged when each of the remaining factors were removed (FIG. 8B). Interestingly, ectopic expression of Hb9 was not required for iMN formation (FIG. 8B), demonstrating that, at least in that case, exogenous Hb9 was not simply transactivating its own promoter. Similarly, Isl1 / 2 expression was detected by immunostaining in iMNs (80.6%, n=36), even when the Isl1 retrovirus was omitted from the transduction (FIGS. 1C-D and FIG. 8B).
[0331]Although Lhx3 and Ascl1 seemed necessary for reprogramming, they were not sufficient to induce motor neuron formation (FIG. 8C). However, when Lhx3 was combined with the three iN factors (Ascl1, Brn2 and Myt1l), a modest number of Hb9:: GFP+ cells were detected (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| resting membrane potential | aaaaa | aaaaa |
| resting membrane potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com