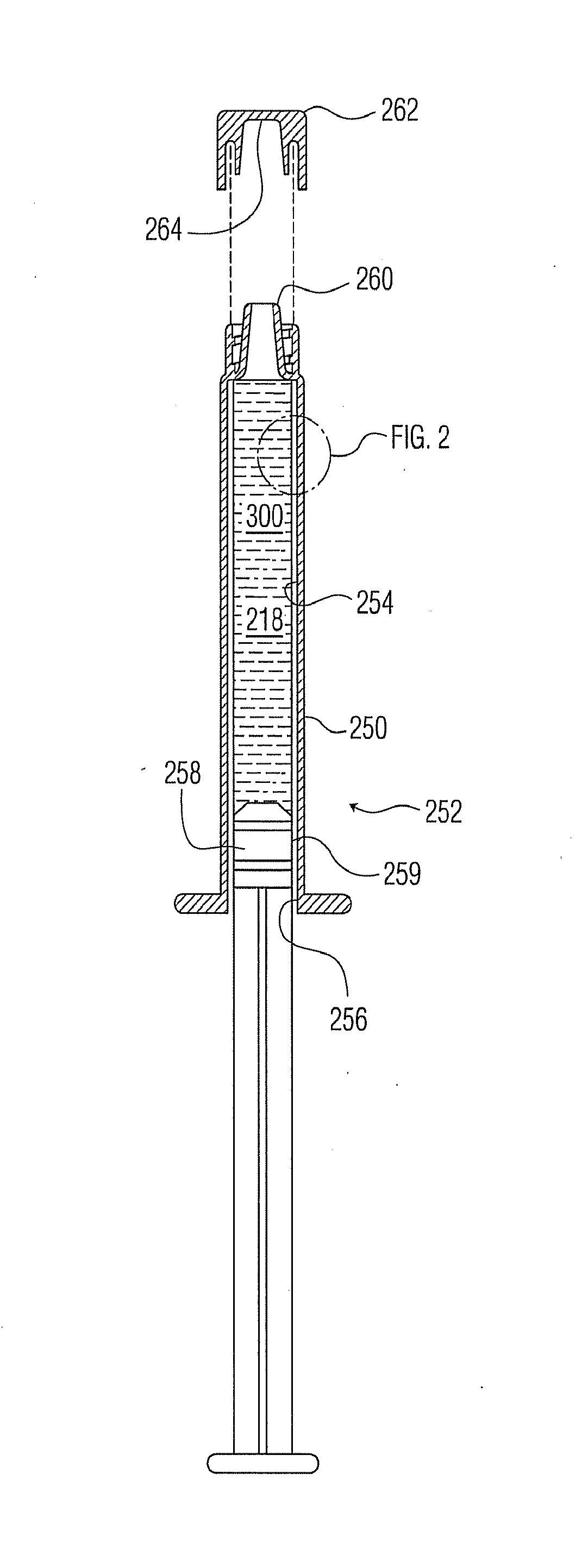
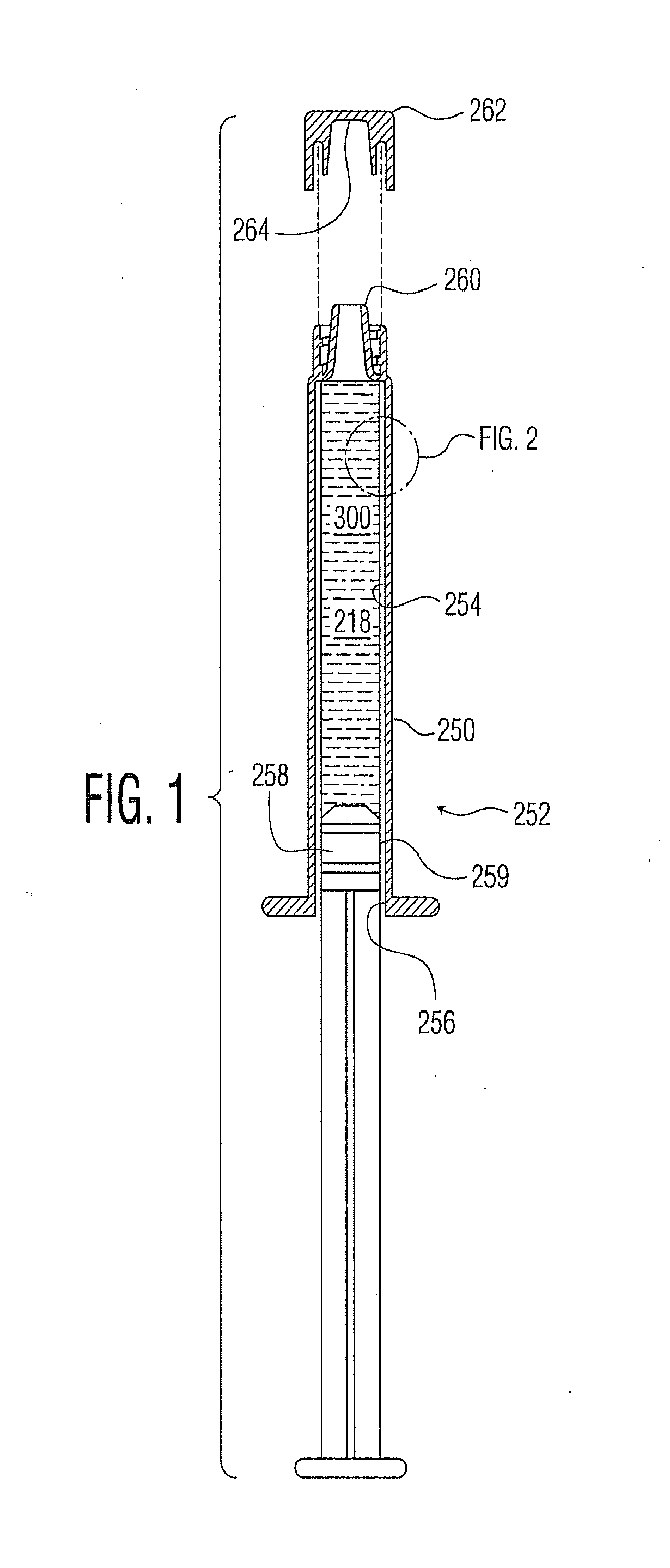
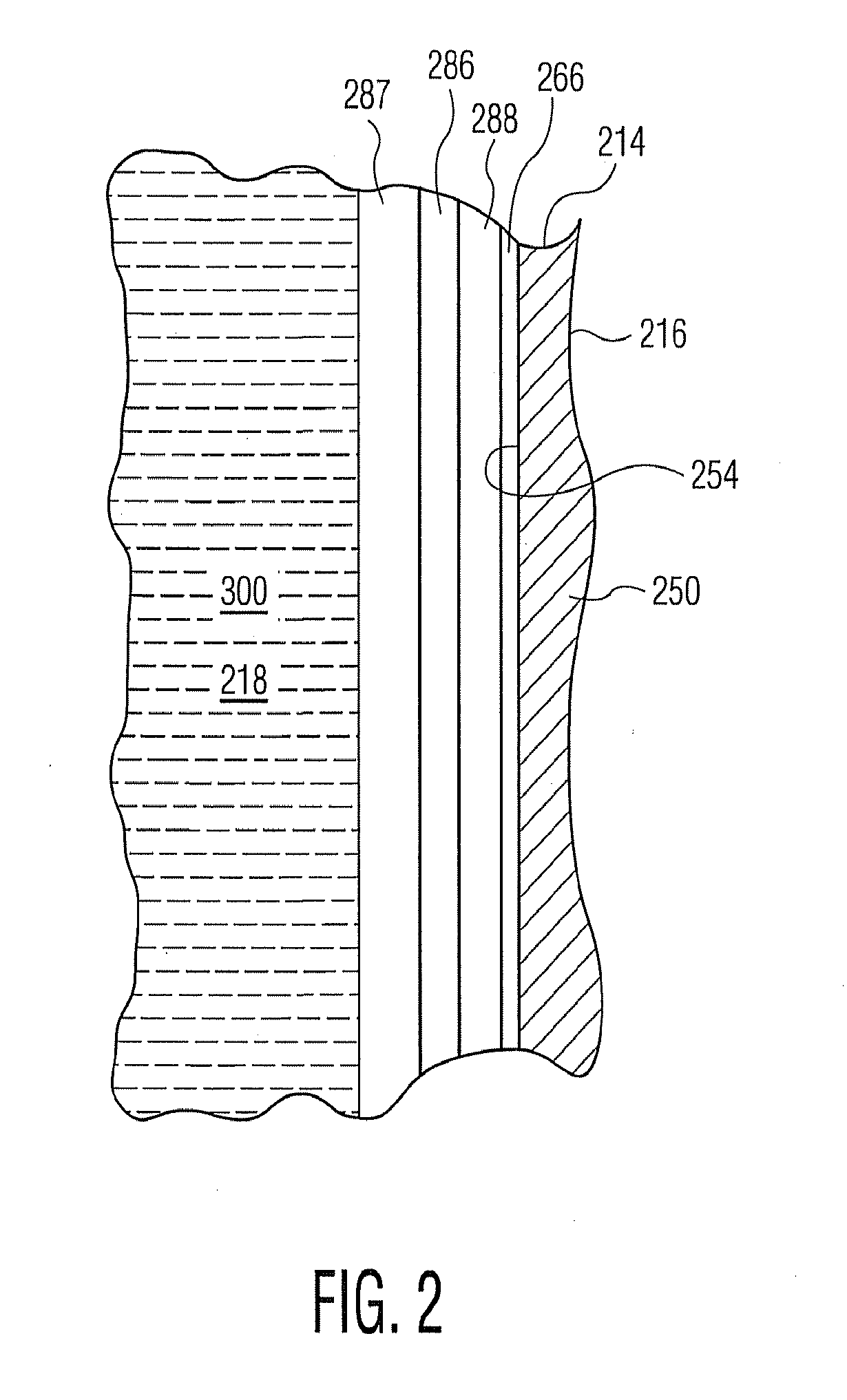
Plasma or CVD pre-treatment for lubricated pharmaceutical package, coating process and apparatus
a technology of lubricating pharmaceutical packages and plasma or cvd, applied in the direction of infusion syringes, surgery, other medical devices, etc., can solve the problems of glass pharmaceutical packages or other vessels that are prone to breakage or degradation, glass particulates may enter the drug, glass-forming processes that do not yield the tight dimensional tolerances required, etc., to improve lubrication, improve lubrication, and reduce sliding friction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Protocol for Coating Syringe Barrel Interior with SiOx
[0208]The apparatus and protocol generally as found in U.S. Pat. No. 7,985,188 were used for coating syringe barrel interiors with an SiOx barrier coating or layer, in some cases with minor variations. A similar apparatus and protocol were used for coating vials with an SiOx barrier coating or layer, in some cases with minor variations.
Protocol for Coating Syringe Barrel Interior with Primer Coating or Layer
[0209]Syringe barrels already interior coated with a barrier coating or layer of SiOx, as previously identified, are further interior coated with a primer coating or layer of SiOxCy as previously identified, generally following the protocols of U.S. Pat. No. 7,985,188 for applying the lubricity coating or layer, except with modified conditions in certain instances.
Protocol for Fi (Breakout or Initiation Force) Measurement
[0210]Convenient methods for measuring the breakout or initiation force required to initiate travel of a p...
examples 1-3
[0221]Syringe samples 1-3, employing three different primer coatings or layers, were produced under the following PECVD conditions:[0222]OMCTS—2.5 sccm[0223]Argon gas—7.6 sccm (when used)[0224]Oxygen 0.38 sccm (when used)[0225]Power—3 watts[0226]Power on time—10 seconds
[0227]Syringe 1 had a three-component primer coating or layer employing OMCTS, oxygen, and carrier gas. Syringe 2 had a two component primer coating or layer employing OMCTS and oxygen, but no carrier gas. Syringe 3 had a one-component primer coating or layer (OMCTS only). The primer coatings or layers produced according to these working examples are contemplated to function as protective coatings or layers to increase the shelf life of the vessels, compared to similar vessels provided with a barrier coating or layer but no primer coating or layer.
examples 4-6
[0228][03] HMDSO was used as the precursor in Examples 4-6. The results are shown in Table 1. The coatings produced according to these working examples are contemplated to function as primer coatings or layers, and also as protective coatings or layers to increase the shelf life of the vessels, compared to similar vessels provided with a barrier coating or layer but no primer coating or layer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thick | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
| inner diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com