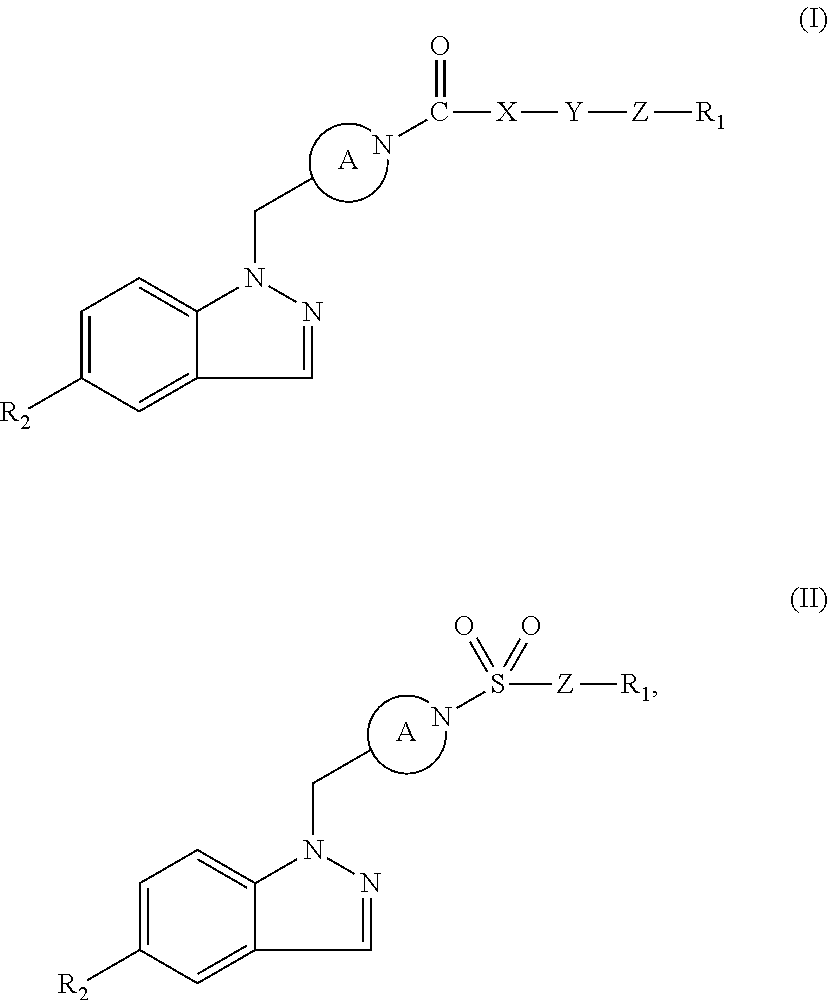
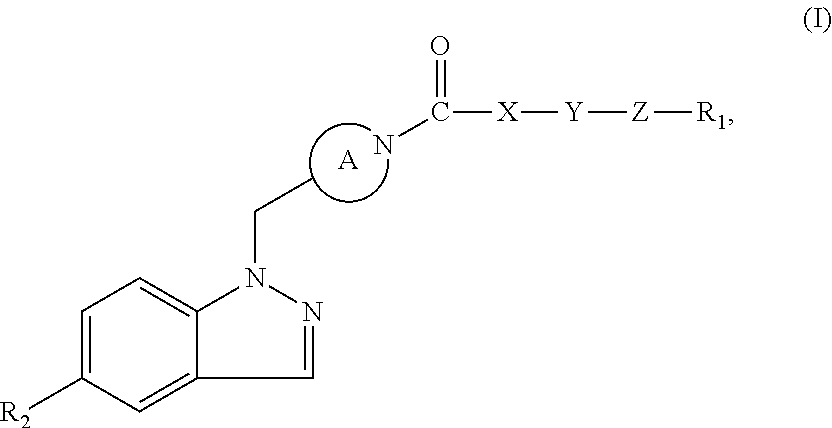
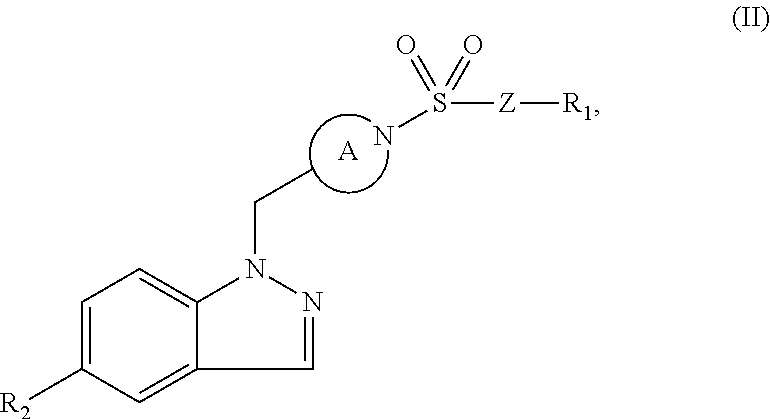
Indazole retinoic acid receptor-related orphan receptor modulators and uses thereof
a technology of orphan receptor and indazole, which is applied in the field of retinoic acid receptorrelated orphan receptor (ror) regulated diseases and disorders, can solve problems such as relative imbalan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 3-phenyl-1-(4-((5-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-4-yl)-1H-indazol-1-yl)methyl)piperidin-1-yl)propan-1-one
[0317]
[0318]3-Phenylpropanoyl chloride (0.115 g, 0.68 mmol, 1.2 eq) and triethylamine (0.127 mL, 0.90 mmol, 1.66 eq) were added to a stirred solution of 1-(piperidin-4-ylmethyl)-5-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-4-yl)-1H-indazole (0.200 g 0.547 mmol, 1.0 eq) in dichloromethane (10 mL) at 0° C. and the mixture was stirred at room temperature for 2 h. After completion of the reaction (monitored by TLC, 100% ethyl acetate, Rf=0.5), chilled water was added and the mixture was extracted with dichloromethane. The organic extract was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel 100-200 mesh, elution with 100% of ethyl acetate to obtain 3-phenyl-1-(4-((5-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-4-yl)-1H-indazol-1-yl)methyl)piperidin-1-yl)propan-1...
example 2
Synthesis of 2-cyclohexyl-1-(4-((5-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-4-yl)-1H-indazol-1-yl)methyl)piperidin-1-yl)ethanone
[0319]
[0320]Using the experimental procedure described in example 1 using 2-cyclohexylacetyl chloride (0.110 g, 0.68 mmol, 1.2 eq), triethylamine (0.126 mL, 0.89 mmol, 1.66 eq) and 1-(piperidin-4-ylmethyl)-5-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-4-yl)-1H-indazole (0.20 g, 0.54 mmol, 1.0 eq) in dichloromethane (10 mL), 2-cyclohexyl-1-(4-((5-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-4-yl)-1H-indazol-1-yl)methyl)piperidin-1-yl)ethanone (0.150 g, 56%) was obtained as a white solid. LCMS purity: 98.33%; (ES+): m / z 490.60 (M+H+); tr=2.30 min.
example 3
Synthesis of 1-(4-((5-(1H-pyrazol-4-yl)-1H-indazol-1-yl)methyl)piperidin-1-yl)-3-phenylpropan-1-one
[0321]
[0322]To a solution of 3-phenyl-1-(4-((5-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-4-yl)-1H-indazol-1-yl)methyl)piperidin-1-yl)propan-1-one (0.20 g, 0.40 mmol, 1.0 eq) in MeOH (10 mL) was added p-toluenesulfonic acid (0.471 g, 2.73 mmol, 5.0 eq) at room temperature and the mixture was stirred at for 6 h. After completion of reaction (monitored by TLC, 100% Ethyl acetate Rf=0.40), methanol was removed under reduced pressure, chilled water was added and pH adjusted to 7 with saturated aqueous NaHCO3. The mixture was extracted with ethyl acetate, the ethyl acetate layer was washed with water followed by brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was triturated with diethyl ether to afford 1-(4-((5-(1H-pyrazol-4-yl)-1H-indazol-1-yl)methyl)piperidin-1-yl)-3-phenylpropan-1-one (0.098 g, 59%) as an off white solid. LC-M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com