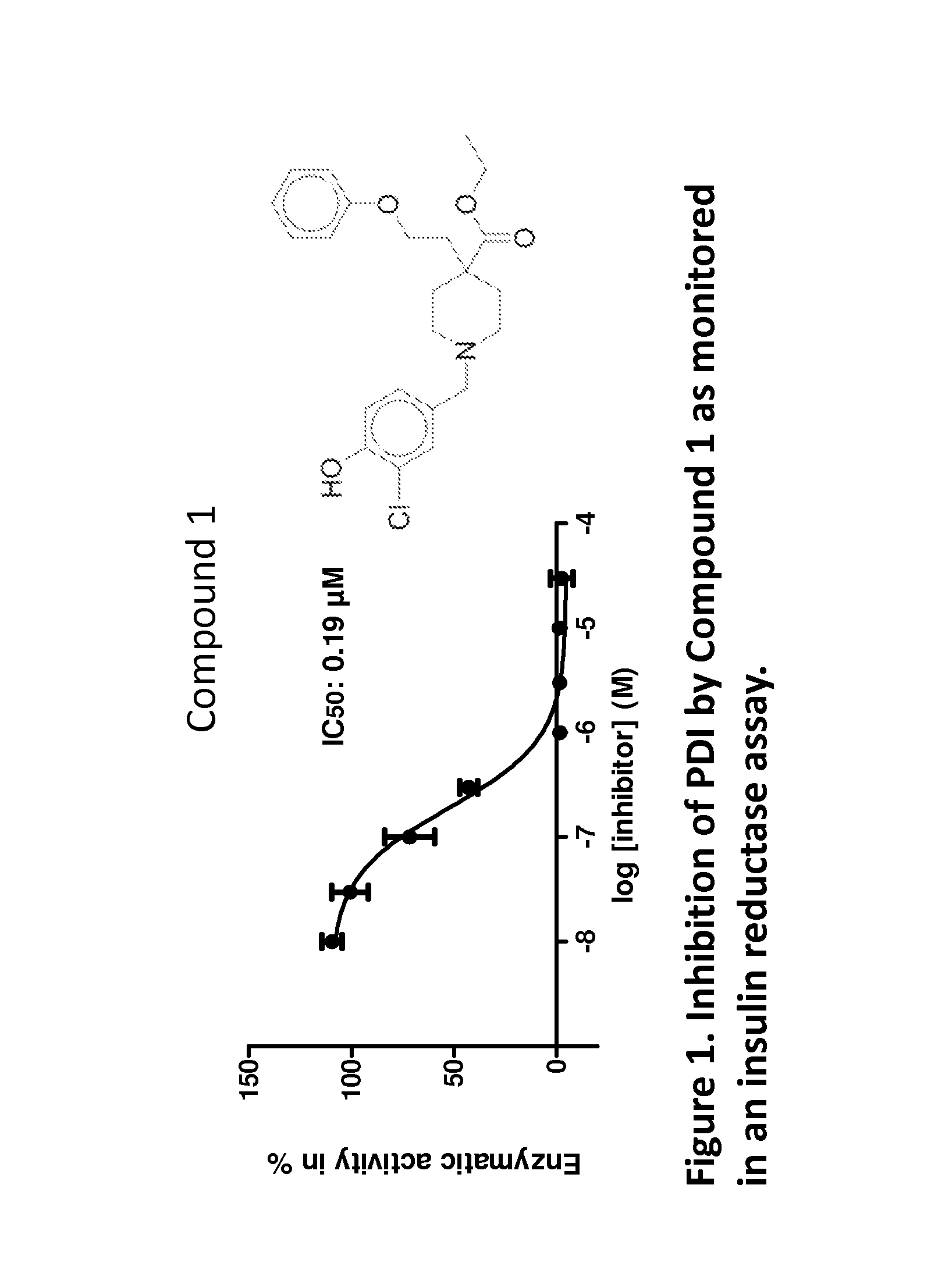
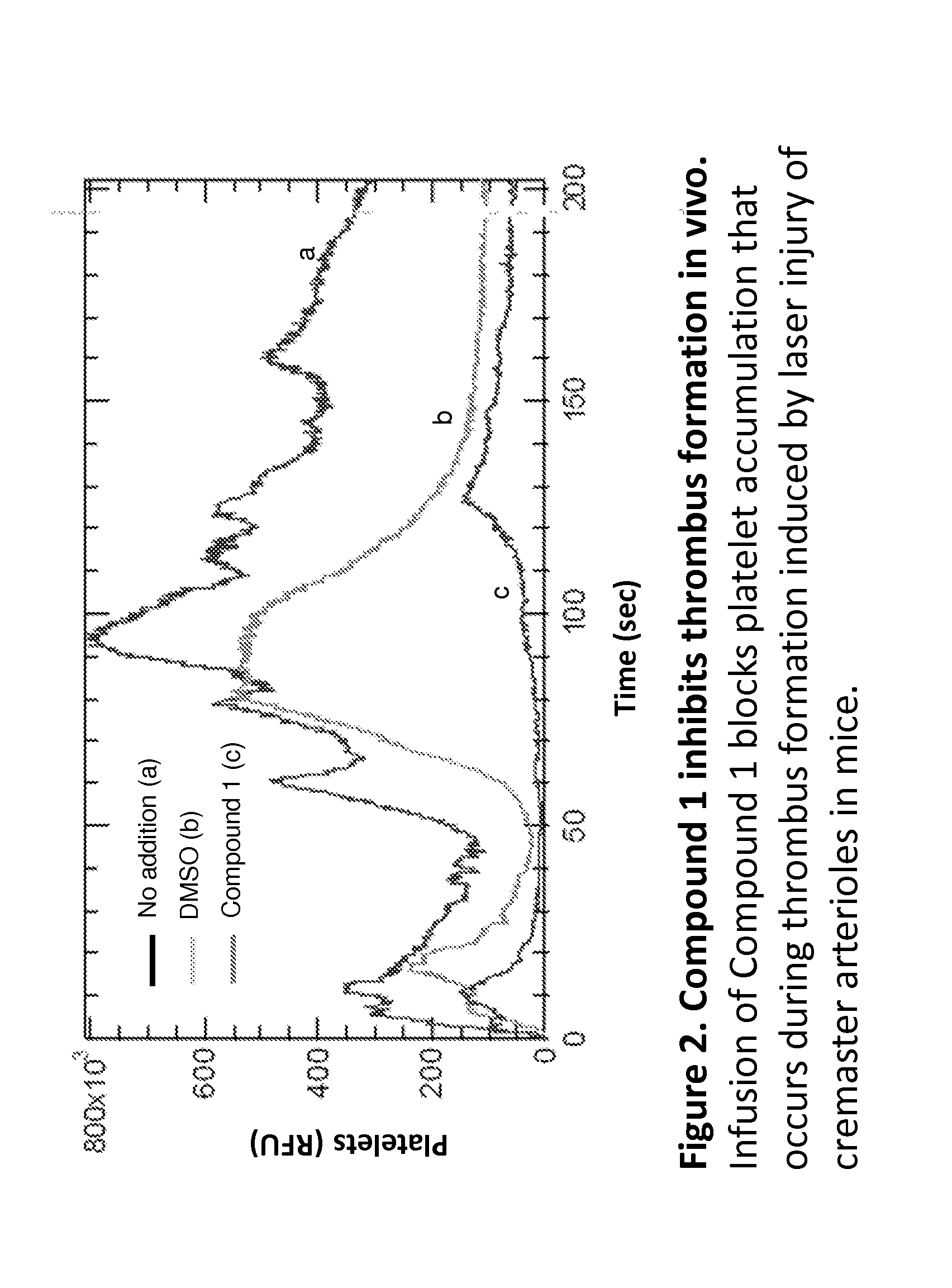
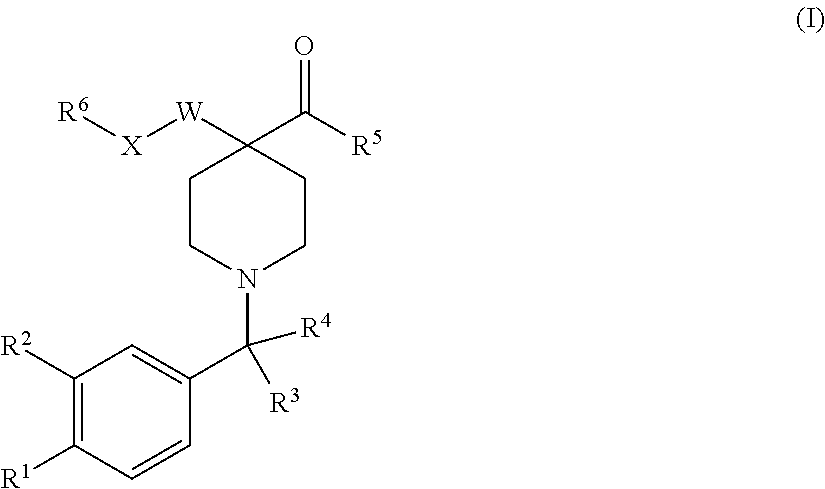
Compounds and compounds for use in methods for treating diseases or conditions mediated by protein disulfide isomerase
a technology of protein disulfide isomerase and compound compound, which is applied in the direction of biocide, drug composition, extracellular fluid disorder, etc., can solve the problems of morbidity and mortality, heart attacks and strokes,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1a
Preparation of Ethyl 1-(3-chloro-4-hydroxybenzyl)-4-(2-phenoxyethyl)piperidine-4-carboxylate (1)
[0067]
[0068]4-(allyloxy)-3-chlorobenzaldehyde (8a): To a solution of 3-chloro-4-hydroxybenzaldehyde (7a, 5.0 g, 31.9 mmol) in anhydrous DMF (20 mL) at rt under nitrogen atmosphere was added K2CO3 (13.2 g, 96.0 mmol) in one portion followed by addition of allyl bromide (4.2 mL, 47.9 mmol). The mixture was stirred at 65° C. for 18 hr. The mixture was cooled down to rt followed by addition of water (100 mL). The mixture was extracted with ethyl acetate (3×80 mL). The combined organic layer was washed with brine (80 mL), dried over Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified by column chromatography over 80 g of silica and eluted with ethyl acetate / hexane (0-30%) to provide the 4-(allyloxy)-3-chlorobenzaldehyde (6.1 g, 97% yield) as colorless oil. 1H NMR (300 MHz, CDCl3): δ 9.84 (s, 1H), 7.91 (s, 1H), 7.76 (d, J=8.4 Hz, 1H), 7.03 (d, J=8.3 Hz, 1H),...
example 1b
Preparation of Isopropyl 1-(3-chloro-4-hydroxybenzyl)-4-(2-phenoxyethyl)piperidine-4-carboxylate (11a)
[0074]
[0075]Iso-propyl 1-(4-(allyloxy)-3-chlorobenzyl)-4-(2-phenoxyethyl)piperidine-4-carboxylate (10a): To a solution of ethyl 1-(4-(allyloxy)-3-chlorobenzyl)-4-(2-phenoxyethyl)piperidine-4-carboxylate (6a, 1.1 g, 2.4 mmol) in MeOH (15 mL) was added LiOH (0.58 g, 24.02 mmol) in water (1.5 ml) at rt. The mixture was stirred at rt for 48h. The reaction mixture was then diluted with water and extracted with ethyl acetate. The combined organic layer was washed with brine and dried over Na2SO4 and concentrated under reduced pressure. The crude product mixture was used in the next step without further purification. To a solution of crude 1-(4-(allyloxy)-3-chlorobenzyl)-4-(2-phenoxyethyl)piperidine-4-carboxylic acid (0.2g, 0.47 mmol) in anhydrous DCM (5 ml) at 0 ° C. under nitrogen atmosphere was added oxalyl chloride (0.08 ml, 0.93 mmol. The mixture was stirred at 0 ° C. for 30 minutes a...
example 1
C: Preparation of Tert-butyl 1-(3-chloro-4-hydroxybenzyl)-4-(2-phenoxyethyl)piperidine-4-carboxylate (12a):
[0077]
This compound was prepared similarly to compound 11a by following the procedures in EXAMPLE 1B replacing iso-propylalcohol with tert-butyl alcohol in the reaction of compound 6a. 1H NMR (300 MHz, CDCl3): δ 7.30-7.23 (m, 3H), 7.11-7.08 (m, 1H), 6.96-6.890 (m, 2H), 6.88-6.82 (m, 2H), 3.96 (t, J=7.2 Hz, 2H), 3.36 (s, 2H), 2.71-2.67 (m, 2H), 2.21-1.94 (m, 7H), 1.62-1.48 (m, 2H), 1.46 (s, 9H). LCMS (ESI+): 446.2 (M+H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com