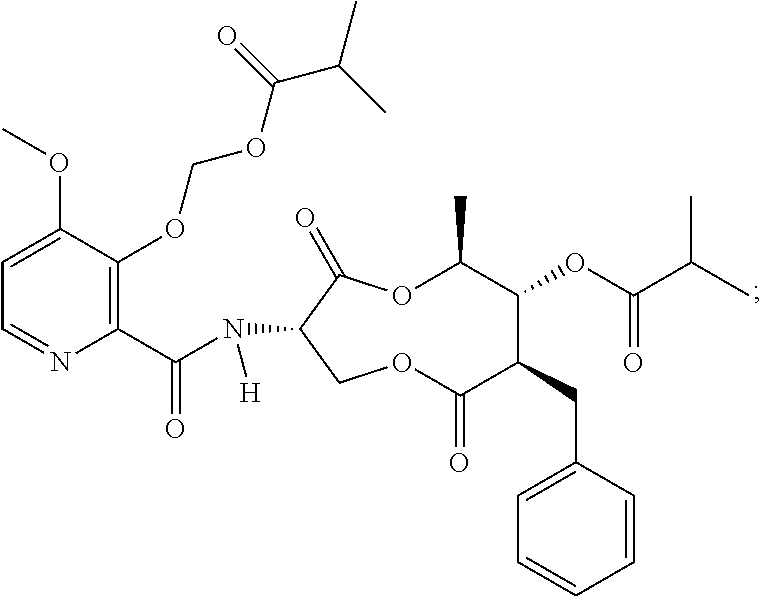
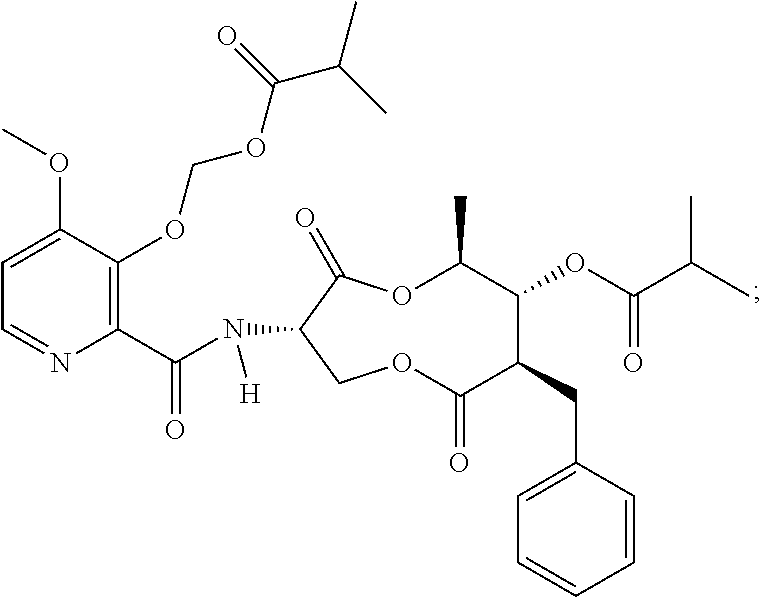
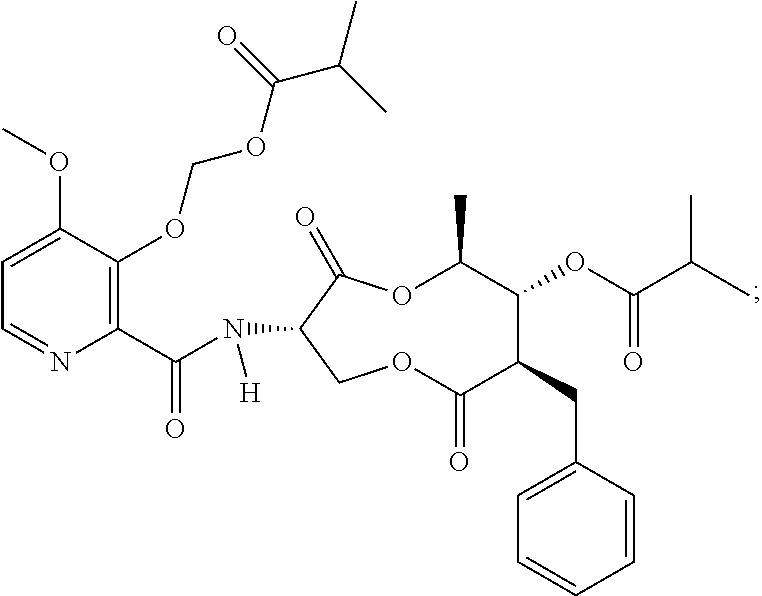
Fungicidal compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Representative Samples of the Described Fungicidal Composition
[0136]
TABLE 1Fungicidal Compositions Described HereinIngredientRoleAmount (g / L)1st fungicide cmpd.active ingredient1-2502nd fungicide cmpd.active ingredient0-200surfactantemulsifier1-100surfactantemulsifier1-100surfactantemulsifier1-100organosiliconeadjuvant10-100 ketonesolvent0-500acetate estersolvent10-750 N,N-dialkylcarboxamide1solvent10-500 polydimethylsiloxaneantifoam0.01-1 1Also known as an N,N-dimethyl fatty acid amide.
Sample 1:
[0137]An emulsion concentrate comprising Compound A as the active ingredient was prepared using the ingredients in Table 2 and as described in the steps below (indicated values are g per 100 mL formulation):
TABLE 2Sample 1 Fungicidal Composition1st fungicide cmpdCompound A, 85% tech.5.88surfactantNansa EVM 70 / 2E6.00surfactantToximul 83204.50surfactantSynperonic 13 / 104.50organosiliconeBreakthru S2335.00ketonecyclohexanone9.89acetate esterbenzyl acetate46.81N,N-dialkylcarboxam...
example 2
Solubility of Fungicidal Active Ingredients in Organic Solvents
[0145]a) Relative solubility of Compound A and prothioconazole in organic solvents with low solubility in water.
[0146]To prepare an effective EC composition comprising Compound A and prothioconazole the following solvent attributes must be achieved:[0147]Compound A solubility needs to be above 10 wt %[0148]prothioconazole solubility needs to be above 20 wt %[0149]water solubility of solvent candidates should be below about 5 g / L or 0.5% so that good emulsion stability will be achieved when the EC is added to water.
[0150]Test Method:
[0151]The approximate solubility of Compound A was determined by mixing a known mass of the active ingredient with an increasing mass of each solvent at ambient temperature. For example, 0.2 g of Compound A was mixed with 1.38 g of cyclohexanone giving a clear solution comprising 12.6% w / w Compound A. Cyclohexanone was therefore classified as a very good solvent for Compound A as it offered “H...
example 3
Storage Stability of Representative Samples of the Described Fungicidal Composition
[0157]a) Accelerated storage stability study of Compound A in liquid compositions comprising benzyl acetate, AMD 810 and a third solvent.
[0158]The stability of Compound A in a variety of liquid compositions stored at 54° C. for 2 weeks is shown in Table 12. The test compositions were prepared in a manner similar to that described in Example 1 using one or more of Compound A, prothioconazole, pyraclostrobin, benzyl acetate, and AMD 810, and a third solvent chosen from cyclohexanone, acetophenone, 2-heptanone, 2-heptanol, oleyl alcohol or 2-ethylhexanol.
TABLE 12Stability of Compound A in Liquid CompositionsComprising Benzyl Acetate, AMD 810 and a Third Solventafter Storage for 2 Weeks at 54° C.CompoundComposition (wt %)A afterCompoundProthio- Pyraclo- BenzylAMDThirdStorage (%Aconazole strobinacetate810SolventThird Solventretention)14.9——94.1——none85 3.4———96.0—none49 12.0————85.7cyclohexanone96 4.97.4—4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Force | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com