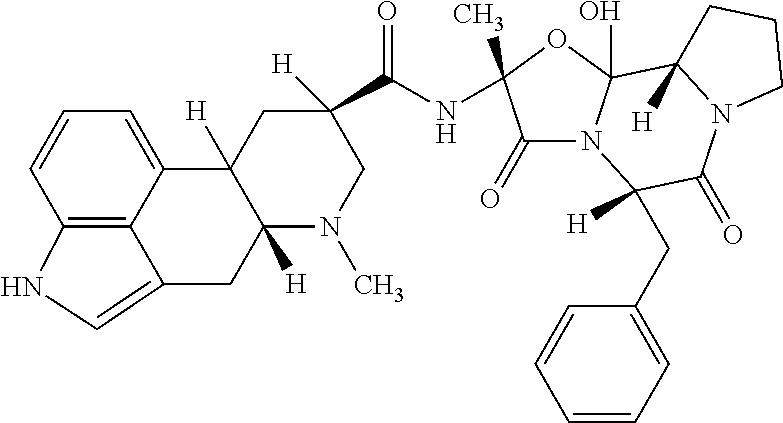
Intranasal dhe for the treatment of headache
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
6.1 Example 1
Preparation of DHE Powder Formulations
[0132]6.1.1 Preparation of 0.1 Mg DHE Powder Formulation
[0133]Powder formulations comprising 0.1 mg DHE were prepared as described herein.
[0134]Materials.
[0135]Dihydroergotamine mesylate (99.7% purity; primary particle distribution: Dv10: 5.3 μm; Dv50: 17.7 μm; Dv90: 69.3 μm; “DvX” refers to the maximum particle diameter below which X % of the sample exists; for example, “Dv10” refers to the maximum particle diameter below which 10% of the sample exists); microcrystalline cellulose, Ceolus® PH-F20JP (nominal particle size: 20 μm, less than 1% retained when sieved through mesh size 400; Asahi Kasei Chemicals Corporation); Microcrystalline cellulose, Ceolus® PH-301 (nominal particle size: 50 μm, less than 1% retained when sieved through mesh size 60 and less than 30% retained when sieved through mesh size 200; Asahi Kasei Chemicals Corporation); Tribasic calcium phosphate (Ca5(OH)(PO4)3; Mallinckrodt Chemicals); HPMC capsule, size 2, ...
example 2
6.2 Example 2
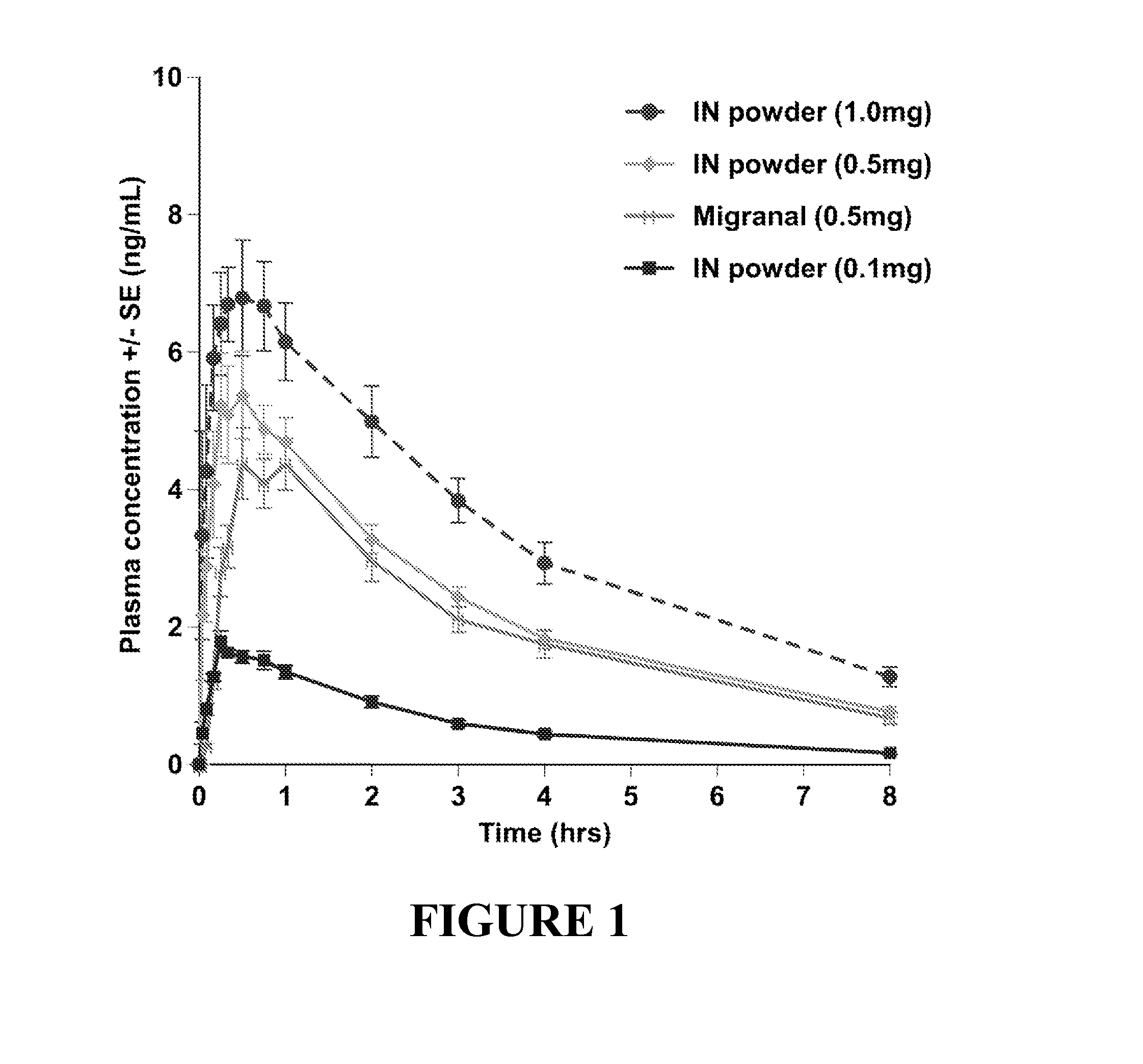
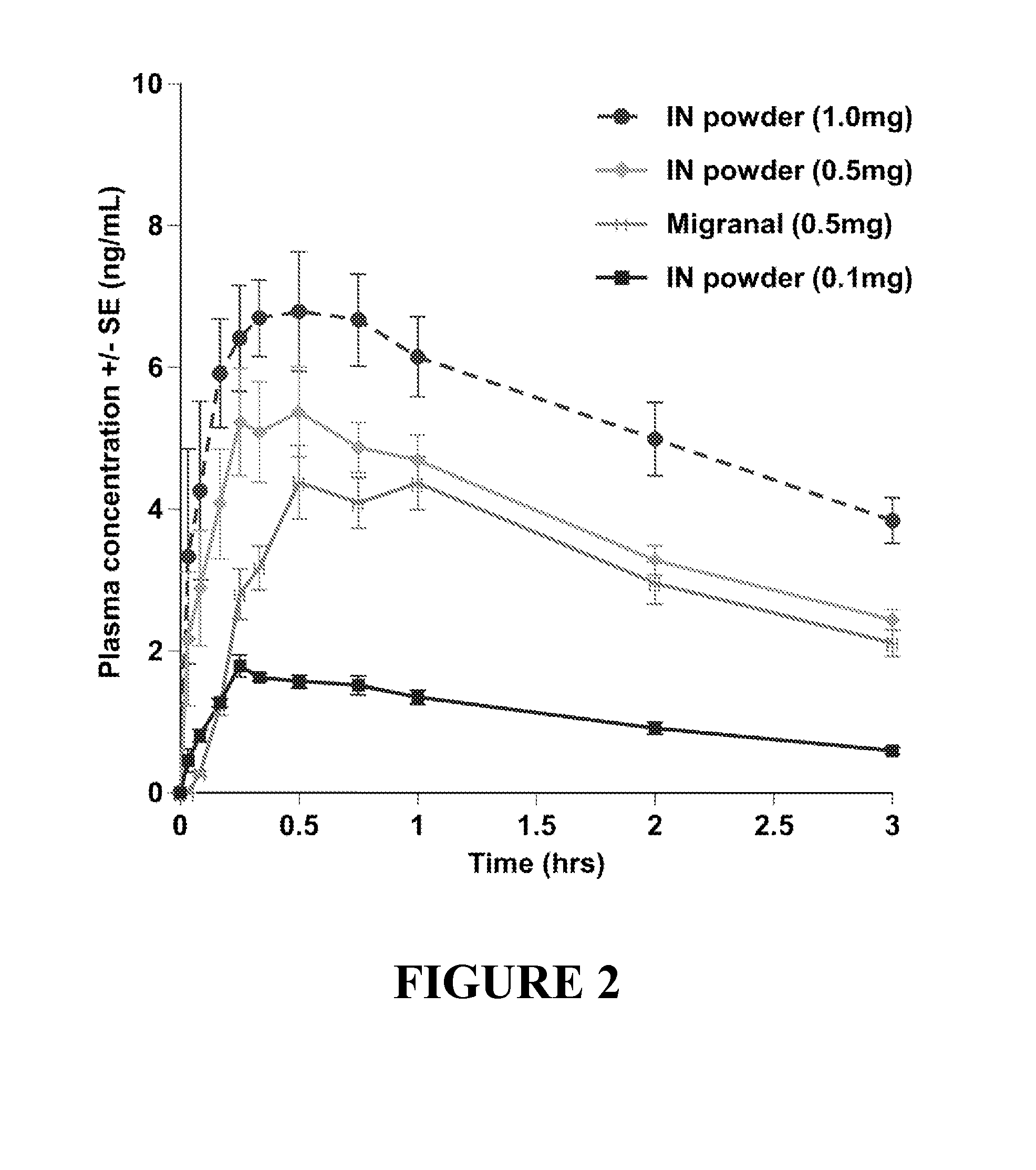
Pharmacokinetic Study of Intranasal Dihydroergotamine Formulations in Primates
[0154]The study described herein is designed to assess the pharmacokinetics of plasma dihydroergotamine (DHE) and 8′-hydroxy DHE levels after intranasal administration using DHE powder formulations described herein, and to compare the pharmacokinetic profiles achieved via intranasal administration of such formulations with those of comparative DHE formulations administered via various dosing routes.
[0155]The study utilizes Cynomolgus monkeys (macaca fascicularis, purpose bred) because the nasal cavity of such monkeys is morphologically similar to that in humans, and is commonly used as an experimental animal.
[0156]Methods.
[0157]Animals.
[0158]Six male Cynomolgus monkeys (macaca fascicularis, purpose bred), 4 to 6 years old are used, following accredited animal welfare standards.
[0159]Test Powder Formulations.
[0160]Powder formulations containing 0.1 mg DHE, 0.5 mg DHE and 1.0 mg DHE, as describe...
example 3
6.3 Example 3
A Randomized, Open-Label, 5-Way Crossover Study to Evaluate the Pharmacokinetics, Dose Proportionality, Safety, and Tolerability of Single Doses of Dihydroergotamine 1, 1.5, 2 and 3 mg Intranasal Powder and Assess the Relative Bioavailability to Dihydroergotamine 1 mg Administered Subcutaneously as a Solution in Healthy Volunteers
[0178]The study described herein is designed to determine the pharmacokinetic profile, dose-proportionality, safety and tolerability of DHE intranasal powder 1 mg, 1.5 mg, 2 mg and 3 mg in young healthy subjects and compare its bioavailability with DHE 1 mg administered subcutaneously as a solution. The pharmacokinetics of DHE and its metabolite (8′-β-hydroxydihydroergotamine; 8′-β-OH-DHE) are characterized in this study.
[0179]Methodology.
[0180]This is a single-center, single-dose, randomized, open-label, 5-way crossover, pharmacokinetic and safety study. Thirty (30) eligible subjects, not less than 40% or more than 60% of either gender, receiv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com